The global challenge of substandard and falsified medical products in the age of Covid-19
Access to quality medicines and medical products is considered one of the fundamental elements for realising the human right to health,26aa9801edb5 but guaranteeing the safety and quality of medical products across the world remains a critical challenge. The manufacture, distribution and sale of poor quality or harmful medical products present lucrative business opportunities for criminals and disingenuous suppliers. The market for falsified pharmaceuticals alone is estimated to reach upwards of $US200 billion annually.55c05f43d25d The circulation of substandard and falsified medical products (SFMPs) in medical supply chains is a drain on state and personal resources, diminishes public trust in health products and institutions, contributes to the global threat of antimicrobial resistance, and in the worst cases can lead to severe illness or death.20c53b4e522f With regards to the ongoing Covid-19 pandemic, the proliferation of SFMPs generates an additional, unique public health risk and threatens effective pandemic control, particularly if new, innovative medical products are quickly rolled out as part of the emergency response. SFMPs are manufactured and infiltrate medical supply chains due to poor quality assurance mechanisms, tiered production, weak regulatory oversight and enforcement, and opaque procurement standards; all areas within a health system that are also at considerable risk to corruption.7bcdab76cc77
The World Health Organization (WHO) estimates that one in every ten medical products in low- and middle-income countries (LMICs) is substandard or falsified.7df0f0aca3e1 Supporting this estimate, a meta-analysis of studies conducted between 1993-2017 to test for SFMPs in LMICs found that 13.6% of all medicines, 19.1% of antimalarials and 12.4% of antibiotics tested were substandard or falsified.60ab05d04e61 In 2015, a WHO study on health commodities for women and children uncovered high rates (64%) of substandard and falsified oxytocin,0647350b01d8 and a study of over 1500 randomly sampled cardiovascular medicines in sub-Saharan Africa found that 16.3% were substandard or falsified (Antignac et al., 2019). Furthermore, the WHO Global Surveillance and Monitoring System (GSMS), which receives, analyses and responds to reports of SFMPs across the world, has recorded SFMPs in over 11 different medicines categories,0dca836c13b9 including vaccines.35a423ce9c8a To date, there is inconclusive data about the prevalence of SFMPs in high-income countries (HICs) but Behner, Hecht and Wahlcb1b69b65527 estimate that even in the most secure markets at least 1% of all medicines in circulation are falsified and the GSMS has confirmed reports of SFMPs received from every region of the world.59212f218d22
The Covid-19 pandemic has exacerbated the global challenge of SFMPs, particularly falsified medical products (FMPs). The oversight mechanisms and anti-corruption infrastructure that are typically relied upon to identify and remove intentionally ineffective, poor quality or harmful medical products from supply chains, such as through detection by national regulatory authorities (NRA) and comprehensive procurement procedures, may be deprioritised or overlooked in order to expedite countries’ responses, or simply overwhelmed due to increases in global demand.
There are many examples across the world of poor quality or harmful products being promoted, procured, and applied for the pandemic response. For example, only eight days after the outbreak of Covid-19 was declared a global pandemic, the International Criminal Police Organization, INTERPOL, released findings from their investigation Operation Pangea XIII into the online sale of substandard and falsified medical products related to the outbreak response. A globally-coordinated team of police, customs and medicines regulators identified and seized over 34 000 SFMPs including personal protective equipment (PPE), such as face masks, as well as substandard hand sanitizers and unauthorised antiviral medications.54f2f682139e Between March 2020 and July 2021, the Medicines Quality Monitoring Globe Index of the Infectious Disease Data Observatory, which collects news reports of SFMP activity in real time,25c8a0ec9c99 recorded 1173 Covid-19 related reports.aafd2595e380 Separate investigations have found personal PPE, diagnostic kits and medicines for patients suffering from Covid-19 to be of poor quality in countries of all income levels.bd4411ddb5a7 This trend was further confirmed through Operation Pangea’s latest operation in May 2021, whereby nine million units were seized, more than half of which were Covid-19 diagnostic kits.214e0571d61d
Additionally, the increase in funds to respond to the Covid-19 pandemic from both governments and international donors overwhelms existing governance structures and processes, such as those for public procurement, which can reduce oversight and risk greater numbers of poor quality or harmful products entering formal supply chains. For example, in Nigeria the government estimates that the cost to vaccinate 70–75% of the population against Covid-19 will be 400 billion Nigerian Naira (over US$ 973 million).2530376ee4c0 Such a sum constitutes approximately 73% of the total national health budget. Coupled with the urgency to provide public services, this will likely strain already exhausted national institutions, leading to further gaps in compliance and oversight.
The Covid-19 pandemic is an unprecedented challenge for all countries across the world. Some argue that we are entering a new ‘age of pandemics’8e4f81d5fcfa making emergency preparedness that can secure the quality of medical products pivotal for the maintenance of public trust in health systems and governance more broadly. Safeguarding against poor quality and harmful medical products also supports effective health resource allocation and increases pandemic response efficiency. Understanding how corruption and corruption risks influence, enable and impact poor quality or harmful medical products in supply chains is greatly needed, not only to aid in guaranteeing product integrity for the Covid-19 response, but also to protect health system supply chains following this pandemic and in preparation for any future disease outbreaks.
Defining substandard and falsified medical products
It is acknowledged that corruption can influence the quality and integrity of medical products entering supply chains,46de8d32a690 however, to the best of the authors’ knowledge, there is no literature that goes into detail on the myriad ways in which this occurs. As a result, it is necessary to set out the working definitions which this Issue applies.
The understanding of the term corruption is set out by the definition from Transparency International as ‘the abuse of entrusted power for private gain’.
The World Health Organization has set out agreed definitions of substandard, unregistered/ unlicensed and falsified medical products.
- Substandard, also called ‘out of specification’, are authorised medical products that fail to meet either their quality standards or specifications, or both.
- Unregistered/unlicensed medical products are those that have not undergone evaluation and/or approval by the National or Regional Regulatory Authority for the market in which they are marketed/distributed or used, subject to permitted conditions under national or regional regulation and legislation.
- Falsified medical products refer to those that deliberately/fraudulently misrepresent their identity, composition or source.
Following from these definitions, the term ‘substandard’ is understood as authorised products of poor quality manufactured or distributed without the intention to deceive. A lack of intention to deceive cannot constitute an abuse of power and substandard medical products, therefore, are not a form of corruption. Where products of unintentional substandard quality, such as expired products, are knowingly supplied this changes the classification to “falsified.”
Unregistered or unlicensed products do not refer to products of questionable quality or integrity and are therefore not part of the investigation of this paper.
Falsified products are those that are of poor quality and deliberately misrepresented. Falsified medical products (FMPs) constitute the focus of this U4 Issue.There are two categories of falsified products identified, namely those manufactured or distributed by legitimate, licensed suppliers within regulated supply chains, and those that are manufactured or distributed by illegitimate or unlicensed suppliers, such as criminals.
Falsified products manufactured, distributed or sold by legitimate, licensed suppliers within regulated supply chains constitute a deliberate abuse of entrusted power and a clear form of corruption. For the purposes of this Issue, such activity will be referred to as “primary corruption.”
Where falsified medicines are manufactured illegitimately there has been no power entrusted and therefore such criminal activity cannot be classified as corruption. However, corruption can facilitate the proliferation of falsified products supplied through illegitimate means. For the purposes of this Issue, this type of activity will be referred to as “secondary corruption.”
Methodology
This U4 Issue applied a mixed methods research approach consisting of a literature review, including secondary sources of quantitative research where relevant, and primary data collection through qualitative research.
The literature review was conducted based on adaptations from recommended rapid systematic review methodologyad61732ca966 to support evidence generation for outbreaks of infections and other health emergencies. This methodology relies on ‘maximising the parallel progression of multiple steps’, which for this U4 Issue includes primary qualitative data collection for validation and triangulation with the literature.
The searched databases/tools are Google Scholar and the LitCovidsection of PubMed. LitCovid is a ‘curated literature hub for tracking up-to-date scientific information about the Coronavirus Disease 2019 (Covid-19)’, which is updated daily.ead4c0a775db Additionally, websites of relevant national, subnational, bilateral, and multilateral agencies concerned with corruption in the health sector and SFMPs were searched. Similarly, the websites of agencies central to Covid-19 response at international and multilateral levels were searched, with additional publications (both peer-reviewed and media reports) recommended by experts in the field. All retrieved documents were subjected to double title and abstract screening and eventual reading of the full-length documents. All searches were conducted on 12 December, 2020. However, additional publications up to 29 March 2021 were incorporated where they added relevant information on emerging issues or where they reinforced or cited documents earlier reviewed.
For primary, qualitative data collection, Focus Group Discussions (FGDs) and Key Informant Interviews (KIIs) applying a semi-structured format were conducted with nine experts: five that contributed to the global perspective, and four that provided expert perceptions on the West African/Nigerian context. Verbal consent to participate was received from all participants. Responses of FGDs and KIIs were transcribed by the authors and an assistant at Bayero University, and cross-referenced by the authors for correctness.
Where no specific reference is given in this Issue, findings were generated from expert interview data. Where direct quotes have been provided, they are clearly ascribed to the interviewee.
The role of corruption in falsified medical products
The first part of this section outlines the drivers of FMPs, and draws connections between these drivers and types of corruption and corruption risks that enable the proliferation of FMPs. The second part of this Issue describes how corruption influences FMPs and how these influences have changed or been exacerbated as a result of the Covid-19 pandemic. A third section highlights existing good practices to prevent, detect and respond to FMPs, especially in the face of the current global health emergency.
Drivers of falsified medical products
To appreciate how corruption is involved in the proliferation of FMPs, it is critical to understand what drives FMPs in the first place. This section highlights the various drivers, identified through the review of the literature and expert inputs, illustrating them using examples from the Covid-19 pandemic. By highlighting the vulnerabilities of the medical supply chain and the mechanisms that allow FMPs to circulate, the ways in which corruption influences FMPs can be better understood.
Supply chain disruption
Poor supply chain management and forecasting, delayed, disrupted or opaque procurement can all contribute to the proliferation of FMPs in national markets.b730db9644ee The Covid-19 pandemic shocked the world and resulted in countries panic buying to increase national stock of needed medical supplies. National procurement systems had to quickly integrate emergency procedures in order to acquire the products needed for the emergency response, such as face masks and other PPE, and equipment like ventilators. At the same time, the supply of all other health products needed for regular health system functioning had to be maintained while global production was placed under severe pressure and additional import/export restrictions were introduced.639977d47936 This unprecedented situation, even in well-resourced countries, resulted in instances of FMPs entering markets through the regulated supply chain.
For example, across the United States, where the threat of the pandemic remains high as does the need for Covid-19-related medical supplies, instances of falsified PPE have been reported. In early 2021, a federal investigation into millions of falsified face masks procured by and supplied to hospitals, medical facilities, and government agencies was launched.2290cd5aafe5 It is argued by Pisani et alc4e7a3a6d3fc that this may be at least partly due to the design of health procurement policies and procedures that often prioritise reducing levels of healthcare spending over product quality.
Product shortages
An emergency situation, such as a disease outbreak, can overwhelm supply chains and disrupt the flow of needed goods to manufacture necessary medical products. Such dynamics drive up the demand for products and can contribute to real or artificial shortages. This scarcity generates a vacuum that is often filled by criminals and even legitimate manufacturers looking to take advantage of the situation for profit. For example, in the very early days of the Covid-19 outbreak there was an enormous increase in demand for PPE and hygiene products, such as face masks and hand sanitizers. In Spring 2020, rising case numbers generated an unprecedented increase in demand and a consequent shortage of face masks across Europe. As a result, governments scrambled to locate and procure face masks in bulk, leading to a number of cases of substandard and falsified products being identified in Hungary, the Netherlands, Switzerland, the UK, and elsewhere.170aa21c2789 Many of the products were manufactured in China, did not meet quality standards and had falsified certification documents.83656c615352 Similarly, amid severe shortages, officials in Thailand reported the seizure of large quantities of substandard face masks, gels, thermometers, test kits, and PPE.706c495895a2 Egyptian authorities also uncovered factories taking advantage of shortages by manufacturing falsified face masks and packaging ethyl alcohol of ‘unknown origin’ intended for sale at inflated prices.e8140eae2661 Furthermore, a 21-country Europol investigation, ‘Operation Aphrodite’, identified and confiscated 27 million substandard and falsified face masks across Europe between December 2019 and July 2020.3cbe6321a960
The opportunity for FMPs can also arise through product shortages that result from burdensome bureaucratic processes to action public funds. For example, the inflexible nature of many public processes in Nigeria led to an inability to maintain services of trusted suppliers and to ensure supply chain integrity. An official from a subnational medical care management agency noted,
‘Subventions come late and are inadequate due to decline in what the government gets (as revenue) due to Covid-19… and the government has bureaucracy. While you can transact with the usual trusted and reliable suppliers, but if they can’t wait indefinitely for payment for all their supplies to you, some of them supply to others who can even pay them cash! And by the time you are ready to buy, the supply is costlier or hard to come by.’
Poor stock management
Many countries, including high-income countries, struggle to maintain good medical supply stock management. A lack of rigorous paper-based or sophisticated digital systems can lead to products expiring in holding warehouses or on pharmacy shelves. Expired products may have lost potency and become less effective or harmful as a result.093a88e221bc A 2018 report commissioned by the former UK Department for International Development (now Foreign, Commonwealth and Development Office) found that repackaging expired pharmaceutical products, including expired donations, giving them new, incorrect expiration dates and reintroducing them into the supply chain is a common practice in some Sub-Saharan African countries.540c7bb7ea00 Even in high-income countries with strong regulatory authorities, there are recurrent instances of expired products being repackaged and sold to consumers. For example, the major pharmacy chain in the United States, CVS, has repeatedly been found to sell expired products including over-the-counter medications and infant formula, with settlements reached in 2009 and 2016, and the most recent reprimand in 2019. A 2008 investigation of CVS in New York State found expired products being sold at 60% of locations.5b6c113599b3
Irrational and unauthorised use
Having an illness or disease is a distressing experience. It brings with it uncertainty and in some contexts the threat of catastrophic financial loss. Covid-19 is a novel disease and research from Switzerland and France indicate that even in settings with well-resourced health systems able to provide comprehensive emergency and intensive care, Covid-19 is three times as deadly compared to seasonal Influenza.a715788a8737 In addition, the contagiousness of the disease and the threat of mutations, the burden of advanced disease on health systems, and the subsequent lockdowns and social distancing measures have had a far-reaching impact on people’s everyday lives and economic stability. The resulting desperation and anxiety from increasingly precarious situations can push people to ignore scientific evidence and advice, distrust public warnings and overlook product quality. It can lead to individuals purchasing products claiming to prevent or treat Covid-19 outside of the regulated supply chain, such as through online vendors, or on grey and black markets.dffdd9fbb9a6
Examples of this include the antimalarials chloroquine and hydroxychloroquine. In early 2020, these medicines were touted by unsubstantiated research to be effective in treating Covid-19. This led to global shortages, which caused the price for the drugs to skyrocket5b9859431f0d and had serious effects for those in need of them for non-Covid-19 indications. For example, the cost of a hydroxychloroquine pack of 20 tablets in Nigeria more than quadrupled from about 2000 Nigerian Naira (~€4.30) to 9000 Nigerian Naira (~€19.50) during the peak of the first wave of the pandemic. In the United States, misinformation about chloroquine and hydroxychloroquine combined with desperation and anxiety increased the demand for the products. Research by the Safe Medicines Coalition that tested hydroxychloroquine samples from the US and foreign online pharmacies found that 15% were substandard or falsified.aaff50ab01ae Shortages due to the heightened global demand also led to an increase in reports of substandard or falsified chloroquine across West and Central Africa.0c890145b468 In the WHO Africa Region, the affected countries include Burkina Faso, Cameroon, Democratic Republic of Congo and Niger, with all reported products identified at patient level.c3a3dccd19db This demonstrates the power of desperation, anxiety and people’s susceptibility to misinformation, and the impact it has on the costs in and integrity of other markets.
Cost of medicines and manufacturing
While the overall out-of-pocket expenditure for health in LMICs is steadily decreasing, it remains proportionally higher as a percentage of overall national health expenditure compared to HICs.bac5559ab59a Increasing costs of healthcare or prices driven up by scarcity can create opportunities for the proliferation of FMPs, as they tend to have lower price points.295a82be9436 There is evidence that patients will seek out lower cost alternatives over the Internet or in unlicensed pharmacies rather than pay full price, especially in countries where there is no state-funded health insurance.a8c4ee783d01 Even if there are no medicines shortages, where out-of-pocket expenditure on health is high, people will be drawn to informal markets for affordability and convenience.86dc6acf7505
Furthermore, looking from the perspective of manufacturers, the desire to maintain or increase profit margins can result in sourcing materials such as active pharmaceutical ingredients (API), excipients or packaging of lesser quality and a lower price tag in order to reduce manufacturing costs. Cheaper ingredients can reduce the effectiveness or increase the speed of degradation of products like pills.99ce03dd5ffc
Misinformation and deception
Consumer ignorance or deliberate misinformation can also introduce and maintain poor quality products in markets. For example, there are two regulatory pathways for hand sanitising products in the European Union – one for classifications of biocidal products and another for cosmetic products.f794bb4fbf61 Biocidal hand sanitisers, which have been advocated throughout the Covid-19 outbreak to reduce the indirect transmission of disease through fomites on surfaces, are required to maintain minimum levels of active substances. Hand sanitiser products registered as cosmetics are not subjected to the same requirements and they often contain inadequate levels of active ingredients to provide a disinfecting effect. Labelling requirements for both product types are not standardised (biocides report by concentration, while cosmetics report by weight).7fbcd5c45e08 This can be very deceiving for consumers unaware of the boundaries of these two regulations and result in their purchasing products that make false or misleading claims.
Because of the Covid-19 outbreak and the subsequent lockdowns placing restrictions on people’s movement and economic activities, sourcing medical products online or through both legitimate and unregulated online pharmacies and suppliers increased in 2020.428b530526f8 The sale of medical products online can offer patients and customers accessibility, convenience, reduced cost, and privacy. However, online sales of medical products and online pharmacies have brought with them considerable challenges to pharmaceutical and medical product regulation and have opened up new opportunities for the distribution of FMPs.5ac96c10d3da For example, research by Mackey et ala85bf9a4beb1 identified spikes in online sales activity on social media platforms that coincided with misinformation put forth by political leaders in the United States for FMPs and products now known to be ineffective against Covid-19. The ease of access through the Internet, apparent legitimacy of online pharmacies, coupled with the limited regulatory capacity can deceive customers, leading them to purchase products they believe to be of high quality.
Limited regulatory capacity
Regulatory authorities are the national and regional bodies responsible for ensuring that medical products meet necessary standards for efficacy, safety, and quality, and for safeguarding distribution in medical supply chains. They are also responsible for educating and informing healthcare professionals and the public about medical product safety and their appropriate use. According to the WHO,5b2fce297298 less than 30% of NRAs worldwide have the capacity to carry out the functions required to guarantee patients’ access to medicines, vaccines and other products that do not cause them harm. Furthermore, few NRAs maintain a policy to publicly release data on instances of SFMPs that could be used to inform and expedite national and international quarantine and recalls.394d276a6b00
The ability of NRAs to detect SFMPs is also undercut by a lack of suitable testing approaches. While there are many low-cost, portable detection devices that have been developed over the last decades for use in low-resource settings, the cheaper a detection device is the less able it is to detect low levels of API or higher levels of impurities in products.d23df85abd1f This means that many SFMPs can move through supply chains undetected. It is not feasible for many NRAs to employ sophisticated and energy-intense testing equipment, such as high-performance liquid chromatography. Even where such equipment does exist, considerable time lags may occur between detection of a suspicious substance and the confirmation of poor quality, leading to significant delays in removing FMPs from the market and issuing consumer warnings.0a9fa3385d77
As already mentioned, Covid-19 has led to an increase in the purchase of medical products on the Internet or through online pharmacies, a trend that is expected to continue.f594ac15277b Since their inception, online pharmacies have been difficult to regulate. While national and international guidelines for such pharmacies do exist, they are so far challenging, if not impossible, to comprehensively implement, particularly with regards to illegal pharmacies.da67122146f7 For example, the latest Operation Pangea investigation in over 90 countries led to more than 113,000 web links regarding falsified medical products, including websites and online marketplaces, being shut down or removed. This was the largest number ever uncovered by an Operation Pangea operation to date.54c6dc69e8a8
Legislation and policy
Government policies and pressure to support local manufacturing may contribute to FMPs, especially during a disease outbreak like Covid-19 when national economies are struggling. Failure to uphold Good Manufacturing Practices (GMP)800f0fca0310 or Good Distribution Practices (GDP)c69adad95707 may be unintentionally or intentionally overlooked to allow local manufacturers to trade medical products both domestically and internationally. This is of particular concern as national regulators often are not required to inspect products for export and regulators of importing countries may not have the needed capacity to routinely assess suspicious medical products.10f98c778ce6 This concern was confirmed in interviews carried out by Pisani et al13aedcf9e0b0 with a Chinese API manufacturer. The manufacturer was well aware that buyers in countries with poorer regulatory capacity or limited resources, such as in Africa, would purchase products of lesser quality, and the manufacturer would even incentivise buyers to take poorer quality products off their hands using attractive discounts.e127a97c4a06
Policies introduced to support the reduction of healthcare expenditures by driving down the costs of pharmaceuticals and medical supplies, including those for Universal Health Coverage, can lead to manufacturers withholding distribution, particularly of expensive products which could be later sold through private, parallel import channels.e5cde4d1875f By withholding products, this generates a gap in the market that purveyors of FMPs will be eager to fill.
Additionally, the low risk of prosecution and weak penalties and sanctions levied on those who are found to be manufacturing, distributing and selling FMPs compared to prosecution for trafficking in narcotic drugs or other illicit substances makes FMPs an attractive and lucrative area for criminal activity.35e85cddb77f For example, in France the maximum penalty for the falsification of medicines is €750,000 and a prison sentence up to seven years.900df3152803 According to the French Penal Code,ed4552e14fee however, the maximum penalty for trafficking in illicit drugs is up to €7.5mil EUR and a prison sentence of ten years.
Challenges to national, regional, and global coordination
Effective control of FMPs requires cooperation at the national, regional, and international levels.dc65f8c8fb0c Presently there are insufficient investments made by national governments, international donors and the private sector into developing the needed capacity and maintaining support for the work of healthcare professionals, national regulators, customs officials, procurement authorities and police to detect and respond to FMPs.6c0586a7e8f1 This enables FMPs to continue to enter and circulate in regulated and unregulated supply chains.
There is considerable reliance on healthcare professionals at the front line and their patients to recognise FMPs and to report them to the responsible authorities. However, detection equipment is often expensive to acquire and operate, cheaper existing methods have poor sensitivity to identify poor quality or contaminated products, and they require additional training to use.5e9fcae987b3 As a result, in a case of adverse side effects or a medicine not having the anticipated treatment effect, healthcare professionals will often simply switch patients to a different product.3ac72e3adb89 In a report from 2017,12bc3716a233 the WHO identified that of the 1500 cases of SFMPs that had been reported to their GSMS between 2012-2017 only 12% had been initiated by healthcare professionals.
Poor cooperation and coordination between HICs and LMICs is contributing to Covid-19 vaccine inequality, widening existing access gaps and driving the market for falsified vaccines and vaccine-related products. Although the Covid-19 Vaccines Global Access (COVAX) Initiative was established in April 2020 and the first vaccines against Covid-19 were administered on 8 December 2020, the first successful shipments from COVAX did not occur until 24 February 2021.d2c8b779df3f This has left many countries that are equally in need of vaccines critically behind. It is anticipated that at the current vaccine rollout rate, some countries in Sub-Saharan Africa will be waiting until 2024 until their populations can be vaccinated.013728f5d38f In Nigeria, the Federal Ministry of Health announced that it is ‘aware of official reports of large-scale fraud and counterfeit Covid-19 vaccines already in circulation’.21ec314e6f00 There have also been reports of falsified Covid-19 vaccines in Mexico, Poland,d096bda75a0e the Philippines,259ae633df60 South Africa,15a7cc4983a6 Uganda6f3d642a5e02 including through the so-called dark web, from which they are widely available to many countries.6879e3102fc6
Compounding this problem, the WHO acknowledges that two thirds of countries do not have systems that can adequately monitor adverse events following immunisation once they have left service delivery points,1ab00d055091 suggesting that these countries are also ill-equipped to detect and respond to falsified vaccines.
There are two critical factors that contribute to the threat of falsified vaccines: 1) vaccine hoarding by countries in the global north, and 2) exorbitant and unaffordable prices of vaccines that limit the quantities that countries with fewer resources can acquire.031234de357b In addition to creating opportunities for FMPs, this also is expected to have a negative effect on global rates of Covid-19-related morbidity and mortality. Modelling carried out by Chinazzi et al230f4420e91f of the estimated reduction in fatalities with uncooperative or disproportional vaccine rollout was 33% compared to 61% reduction in fatalities with a more cooperative allocation of vaccines.
‘Very large numbers of LMICs are left out for vaccines right now. That creates a market for cheaper alternatives, of diverted products. How are these transported and who receives them under what conditions? There is a lot of room for misconduct there’, – Dr Nikos Passas, Professor of Criminology and Criminal Justice; Co-Director, Institute for Security and Public Policy [Interview conducted 10 December 2020].
Corruption, FMPs and Covid-19
The role of corruption in FMPs has yet to be carefully explored. This is likely due to FMPs often involving criminal activity, which itself does not strictly fall within the domain of corruption, but also due to limited investments made in anti-corruption research regarding FMPs generally. Based on available data and the responses from interviews carried out for this U4 Issue, it is clear that corruption is involved in the proliferation of FMPs in medical supply chains in myriad ways. This U4 Issue categorises the various forms of corruption and corruption risks that facilitate FMPs. It highlights the roles of primary and secondary forms of corruption and FMPs (see section on Definitions above), using examples from the Covid-19 pandemic response.
The Covid-19 pandemic has brought with it significant challenges that exacerbate levels of existing corruption, which consequently impacts upon the quality of medical products in supply chains. There have been attempts to draw parallels between the Covid-19 outbreak and other health emergencies, such as the 2013-2016 Ebola outbreak in West Africa, to distil good practices for addressing medical supply chain corruption. However, with regard to FMPs there may be little that one can learn from past experiences. During an interview conducted on 2 February 2021, Sarah Goldsmith, the Head of Procurement Delivery at Crown Agents, said,
‘I have never dealt with anything quite like the Covid-19 crisis. Ebola was much smaller scale - only a few countries. If you looked far enough, you could find supplies and there were no hurdles to exporting those goods. When the Covid-19 pandemic hit, global demand outstripped supply in a number of key medicines, PPE, medical equipment; there were massive shortages. Many of the main manufacturing locations were hit and were either in lockdown with no manufacturing going on, or there were logistical issues, and there simply weren’t the means to move products to end users. And then on top of that you had export bans. It didn’t seem like it at the time, but looking back [Ebola] was much, much easier to deal with than Covid-19.’
Similar observations were echoed by a sub-national medical care management official in Nigeria:
‘When you have a cholera outbreak NEMA [National Emergency Management Agency] is there to help you and maybe one or a few other locations needing that help. But when everywhere is affected and even Abuja [Nigeria’s capital] is among the worst affected [by Covid-19], you can’t count so much on what someone else can do for you. It becomes a new and unfamiliar situation.’
The unprecedented nature of the Covid-19 pandemic has made responding to the crisis more challenging than any other public health emergency in living memory. The full manifestation of how corruption drives and facilitates the manufacture, distribution and consumption of FMPs throughout the outbreak response has yet to be fully determined.
The following provides an overview of the areas of corruption and corruption risks that help facilitate or impact FMPs across medical product manufacturing and distribution, product regulation, procurement, governance, and dispensing. Where relevant, it also provides examples of FMP-related challenges that have been identified throughout the Covid-19 outbreak and illustrates how corruption in this health emergency amplifies FMPs in supply chains.
Manufacturing and distribution
Producing FMPs is not necessarily challenging, especially in poorly regulated environments. It can be a great business to be involved in as it is both lucrative and there is lower prosecutorial risk in FMPs compared to other types of criminal activities, such as trafficking in illicit drugs. There are a number of corrupt practices that impact on quality assurance at the level of manufacturing and distribution that facilitate FMPs entering supply chains.
In legitimate manufacturing there are several types of primary corruption or corruption risks. For example, GMP compliance is required to provide the needed assurance that medical products are manufactured and controlled in accordance with the set standards of their product specifications and is an indication of product quality. Certain companies may also acquire what is referred to as WHO Prequalification, a mark of product quality, efficacy and safety for APIs, excipients and finished products according to global standards. WHO Prequalification is used by agencies of the United Nations and other procurement agencies to inform and expedite medical product purchasing. Corruption may infiltrate GMP or WHO Prequalification, as manufacturers may bribe regulators to overlook poor compliance or grant unwarranted certification. A case in point is the Chinese API manufacturer, Fosun Pharma, which has been accused of submitting fraudulent documents to receive GMP certification and bribing local officials to approve changes to production processes for antipsychotic and chemotherapy medications.3be3ca582f56
Many testing approaches for FMPs are either inadequate or expensive and require considerable training, and most approaches that can be applied in resource-constrained environments are not able to detect if a product has the correct API or substandard levels of an API. Therefore, companies may push the boundaries of GMP requirements and only just fulfil specifications that pass testing, use the wrong APIs, or dip below minimum API values for products to be used in countries with poor testing capabilities.
This is an important primary corruption risk, especially in the face of possible disruption to the supply of APIs and excipients in key markets, such as India and China.fb801c64f3b3
‘We have not had too many acute shortages yet with API…[but] if there is an acute shortage of drugs then that will lead to a lot of [falsified] drugs flooding the market. What will follow is corruption at the level of procurement, because people will be diverting products to other spaces.’
– Dr Timothy Mackey, Director of the Global Health and Data Policy Institute and Co-Founder of S-3 Research [Interview conducted 4 January 2021].
This risk can be coupled with increasing economic pressures on manufacturers and national pressures to reduce the cost of public procurement for the outbreak response. Companies looking to increase or just maintain their profit margins may take advantage of poorly resourced or overwhelmed regulatory oversight, or limited import-country testing capacities, to intentionally reduce the quantities of API or allow for greater quantities of impurities in manufactured products, making them ineffective or even harmful.
Looking at product distribution, a classic form of distribution-related, secondary corruption is the outright interception and theft of medical products in transit for sale on the grey or black markets. Particularly at risk are countries with limited regulatory capacity to detect these products in their supply chains. Police and customs officials responsible for safeguarding the entry and passage of pharmaceuticals and other medical products may be persuaded through bribes or collusion to allow FMPs from legitimate or criminal suppliers to enter the supply chain.2a833d8851f3
In situations where there is high demand for medical products coupled with growing scarcity, as is being observed in the Covid-19 outbreak, there are more opportunities for abuse. Dr Timothy Mackey says,
‘...What we are going to see is [those] that are manufacturing these products have the ability to manufacture them because of underlying corrupt acts within their country or jurisdiction…Where are these products coming from and how are customs authorities allowing them to be exported out of the country?... It will not just be quick individual criminal acts, but systematic corruption or gaps in the regulatory system that are not addressed.’
This is illustrated in a case from February 2021, whereby imports of poor quality, unsterile needles and syringes in Nigeria were reported. Representatives from the Nigerian Senate acknowledged that customs authorities were not carrying out their duties to levy a tax aimed at curtailing importation of poor-quality products.cab8e5f7c932 This is not only a challenge for LMICs. Also in February 2021, falsified N95 face masks intended for first responders were sold to hospitals, medical facilities and government institutions in at least five states in the United States.032b82f9b551
When asked which companies are most likely to engage in corrupt activities to intentionally undermine product quality, Dr Nikos Passas indicated, ‘for the more prestigious and established companies, [manufacturing FMPs] is a risk managed by compliance, or one they are not willing to take because brand, reputation and protection is undermined if they do’.
This suggests that the manufacture of FMPs is most likely to be carried out by criminals, companies that are confident they will not be found out, or companies who are not concerned with reputation damage, such as newly founded, opportunistic companies looking to enhance temporary profit.
Regulation
Overall, there is insufficient attention and investments for the regulation of medical products. NRAs and regional regulatory authorities (RRAs) are often underfunded, which negatively impacts on their effectiveness. Where they are adequately funded, they often rely heavily on funding from industry stakeholders, which can lead to conflicts of interest that undermine their legitimacy and integrity. For example, the European Medicines Agency (EMA), responsible for the harmonised evaluation and oversight of medicines for countries of the European Union, receives 86% of its funding from industry fees and charges for services such as market authorisation.b4c71e9d7c8b
NRAs may have conflicts of interest or collude with licensed manufacturers, or accept bribes to approve FMPs or grant fraudulent GMP certification. NRA employees also may collude with manufacturers of FMPs and grant market registration or approval without needed documentation demonstrating product quality or safety. An example of this is the company, Sinovac BioTech, which developed the Sinovac vaccine for Covid-19. In 2016, the company’s CEO, Yin Weidong, admitted to paying bribes amounting to more than $83,000 to Chinese regulatory officials between 2002 and 2011 in order to fast-track vaccine approvals before there was sufficient evidence of product safety and efficacy.0ae64120ccfc This also occurred when Sinovac was leading the development of vaccines for SARS, avian flu and swine flu.262e2d8046df The CEO is quoted saying he ‘could not refuse’ the regulator’s bribery requests.b03ed8f90b76 This case follows from a history of corrupt behaviour, with records showing at least 20 officials or hospital staff receiving bribes from Sinovac employees between 2008-2016.4b8a2f3d4bc8
Furthermore, national commitments to promote local manufacturing can create perverse incentives whereby local companies may collude with NRAs for GMP qualifications or market approvals, as was seen in the Sinovac case illustrated above.
The Covid-19 pandemic undoubtedly stretches the already limited resources of NRAs even further. With it comes an influx of several medical products to be approved and overseen, which may be easily manufactured at a lower quality, such as face masks, diagnostics, medicines, and vaccines. Almost immediately after the early warning signs of the pandemic, suspicious products, such as Covid-19 diagnostic testing kits, hydroxychloroquine and “Covid cures” were reported.c125dd7d08b9 This is particularly concerning for vaccines to protect against Covid-19. ‘It is not difficult to put something else in the vial that looks [the same] and it is very hard to do any testing on the spot’, says Dr Nikos Passas.
Even the best resourced regulatory authorities are strained due to the administrative requirements of the Covid-19 response. Their limited capacity increases the risk of FMPs’ proliferation and related corruption. This is demonstrated in the EMA’s issuance of an automatic extension for GMP manufacturing authorisations, GDP certificates and time-limited wholesale authorisations until the end of 2021.6b099b84e4af Manufacturers and distributors may abuse this extension and seek to widen profit margins at the expense of product integrity and quality.
For instance, Nigeria’s regulatory capacity, already sub-optimal prior to the Covid-19 outbreak, was further jeopardised due to widespread restrictions on movement and stay-at-home orders for essential workers in regulatory agencies and their partners. An NRA official in Nigeria said,
‘There was a restriction of movement for all staff on [salary grade] level 14 and below. If you do not have the bulk of your staff around, even what you were doing before now becomes harder. You cannot enforce regulations from home…The police were on ground, but police are not able to detect [poor quality] drugs.’
Procurement
Public procurement in the health sector is highly vulnerable to corruption and is a main avenue for FMPs to enter into legitimate supply chains.e12f3d8e6c03 Procurement officials may solicit bribes from manufacturers wanting to participate in tenders or collude with them to take a cut of a procurement contract that includes products that are falsified. This occurred in 2019 in Zambia, where the Ministry of Health awarded a tender valued at $US17 million to an unregistered company for health kits that were of poor quality and unsafe to use.4d252beaafde
Generally speaking, lack of transparency throughout the procurement process increases the risk of corruption and abuse. Without adequate transparency, data about the quality-related specifications of products to be procured can be manipulated to allow for poor quality or falsified products, or favour suppliers that have bribed or colluded with procurement officials to secure tenders.
The same can be said for a lack of transparency around the pricing of medical products. There is evidence that some FMPs are as expensive or more expensive than quality products,360a981a7dfc however, the typical draw of FMPs is that they are less expensive because they do not contain costly ingredients. Without pricing transparency, it is not possible to compare with other markets to detect possible anomalies. Results from a report by Transparency Internationale06a0af40cd4 highlight the severe lack of transparency in Covid-19 vaccine contracts and stark disparities in cost across different countries. Indeed, some LMICs have been charged higher prices that HICs, limiting the volumes they can procure for their populations and increasing the risk of falsified vaccines to be introduced.
Furthermore, opaque procurement procedures, coupled with a lack of transparency of the beneficial ownership of companies, can hide conflicts of interest between procurement officials, their family members or friends, with medical product manufacturers. They may abuse procedures such as direct appointments or rig procurement tenders and bidding to preference manufacturers with which they have connections, regardless of the company’s ability to present GMP/GDP certification or fulfil other regulatory requirements. For example, in Kenya, the legal frameworks surrounding conflicts of interest and due diligence requirements for public officials do not prevent them from doing business, and it is not uncommon for public officials to be connected to companies incapable of fulfilling contract specifications.df68bdaee26c In May of 2020, Kenya’s Minister for Health admitted that the country had been importing poor quality PPE and it was only after this scandal came to light that the decision was taken to use quality-assured, locally manufactured PPE.d2a096c839d6
Health governance
At the governance level, there are additional corruption risks that can contribute to the proliferation of FMPs. Politicians may have personal or affiliate interests in companies that manufacture FMPs, creating conflicts of interest in medical products’ procurement. Like regulators or procurement officials, they may also solicit bribes or collude with manufacturers and criminals to allow their products into the supply chain.
Leaders may prioritise themselves, their families, friends and allies in the rollout of new treatments or vaccines, thereby generating greater product scarcity for those most in need of preventive and treatment services. This added scarcity widens gaps in access and further incentivises FMPs entering markets. Examples of this have been reported all over the world with members of royal families, athletes, and journalists jumping the vaccine priority queues.aedbc43e9847 In Argentina, the Health Minister was forced to resign following evidence that his office had granted those with connections to him early access to the vaccine,b4021e315c92 and in Peru, a scandal emerged in February of 2021 in which over 450 people (mostly public officials) received vaccines intended for clinical trials. Included among those vaccinated were the Minister and Vice-Ministers of Health.b6bc67ad7309
Most egregiously, those in positions of power may fail to appropriate funds or devolve power to regulatory and customs authorities or law enforcement to carry out their oversight duties. In some instances, there may also be confusion among stakeholders on how to enforce laws against FMPs and the funding available for this to happen. This was the case in Pakistan in 2012. A regulatory authority was established following a major incident involving locally manufactured, falsified heart medication that led to nearly 150 deaths; however, the new authority was set to be severely underfunded and therefore unable to carry out its functions.154f3dcffb02 Since then, the Drug Regulatory Authority of Pakistan has been called out by various organisations for engaging in various types of corruption, particularly around excessive drug pricing. Issues of FMPs in Pakistan remain a serious public health problem.c1b4b9e6b3cc
Medical product dispensing
It is possible for FMPs to reach patients through formal, regulated channels, including registered pharmacies and healthcare professionals, as well as informal, illegal and poorly regulated channels, such as black and grey markets or through online pharmacies and the dark web. Recently, Interpol’s Operation Pangea shut down more than 100,000 online marketplaces offering falsified medical devices. The most common were Covid-19 testing kits.40a3b3a49dce
Manufacturers and distributors may bribe or collude with healthcare professionals to prescribe FMPs to their patients. In February 2021, two Californian oncologists pleaded guilty for purchasing and prescribing more than $US 1 million worth of unapproved and falsified cancer drugs. Given the public health implications, the sentences they were facing, which included a one year of probation, $US 1.2 million in fines, and forfeitures on behalf of Haematology Oncology, were highly inadequate.ff496c56d555
In environments where wages are low or penalties are not proportional or enforced, healthcare professionals have been found to dispense FMPs. For example, the Institute for War and Peace Reporting649b447c2b90 recorded accounts from patients in Afghanistan that doctors were making a profit off selling poor quality medicines. More recently in India, 12 Covid-19 vaccination drives near the metropolitan area of Mumbai organised by doctors and medical workers were found to be vaccinating patients with saline solution. Upwards of 2500 people paid for and received false injections with those who organised the drives earning over $US 28 000.dcdac8f33d02
Covid-19 has brought with it considerable economic instability, which has had a serious impact on the wages of public officials and healthcare professionals. Low salaries or delayed salary payments can increase the risks that those responsible will overlook or be less likely to report suspicious products and may even demand bribes. Dr Nikos Passas says, ‘if salaries are not paid, that will give an opportunity for some corrupt pharmacist or doctor or other official to allow substandard or falsified vaccines to be administered’.
Healthcare professionals, such as physicians and pharmacists, may tamper with the composition of medical products, compromising their quality in order to increase their own profits. For example, there are accounts in Germany512225516c13 of healthcare professionals diluting cancer medication in order to stretch APIs, while still charging patients or their insurance providers the full price.
It is also possible that healthcare professionals engage in FMP-related corruption on account of being bribed or colluding with criminals to dispense falsified medicines/medical products, or they may otherwise facilitate FMPs in supply chains through their own naivety or opportunistic behaviours. For example, a healthcare professional in the United States was found to be selling empty Covid-19 vaccine vials through online platforms like eBay and Craigslist.7338000f1d81 Vials were advertised as souvenirs and suggested that buyers could have a “piece of history”. These vials in the wrong hands could be refilled and sold as legitimate vaccines, leading to possibly deadly consequences.
Finally, nepotism or favouritism in the dispensing of legitimate health products at the facility level (with or without bribery) are further risks. This disadvantages other populations, creating scarcity, pushing them to seek products on the grey and black markets that may be harmful. For example, since Venezuela started the national vaccination campaign on 18 February 2021, its Transparency International chapter has been flooded with complaints over vaccine queue-jumping by government officials and other powerful groups, leaving the most vulnerable groups – such as healthcare professionals, the elderly, and people with pre-existing conditions – at risk of getting infected and dying from Covid-19.c567dec84262 Given the government’s slow and obscure rollout of vaccines, many have turned to black markets to access Covid-19 vaccines. In Lara state, at least 2,000 people were inoculated with jabs made of boiled water, antibiotics, and analgesics, at a cost of US $100-450 each.52ea8134bec3
Figure 1. Conceptual Model for Corruption in Falsified Medical Products
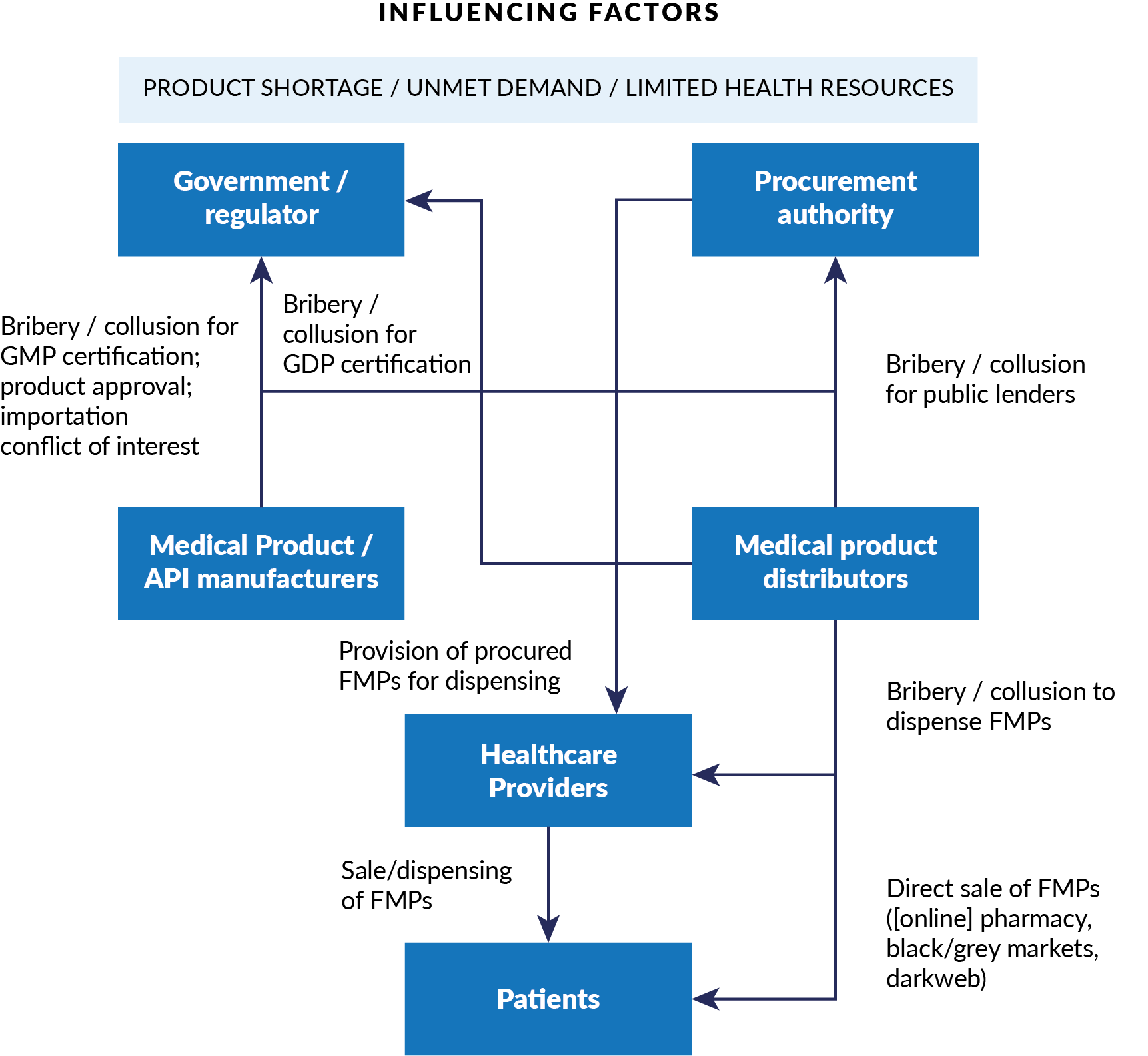
Anti-corruption strategies to address FMPs in health emergencies
‘There is a recognition that this is a global problem and needs a global response. There needs to be a lot more focus put on emergency preparedness. You do not know what you will be responding to, but having processes in place as to what you would do in such a situation and how countries would work together [is needed].’ – Sarah Goldsmith, Crown Agents.
The globalised nature of the medical supply chain necessitates cooperative, global solutions and approaches that prioritise medical product quality and integrity to promote, improve and uphold public health. There are three strategic areas identified to address FMPs in medical supply chains, namely, prevention, detection, and response. The following considers existing approaches and analyses them from the perspective of anti-corruption in order to determine the gaps and formulate recommendations for an improved response.
Corrective strategies should be applied in accordance with sound prioritisation of the FMP-related corruption risks, targeting those risks that are likely to generate the greatest harm to supply chain functioning, medical product integrity and, inevitably, end users. It should be noted that solutions to FMP-corruption may not involve traditional anti-corruption solutions, but rather be embedded within or be a secondary effect of overall supply chain efficiency improvements. Governments and development actors are encouraged to review Wierzynska et al. (2020) for guidance on effective priority setting.
Prevention
Identifying ways to advance and safeguard product quality and prevent FMPs from entering into supply chains is the most powerful weapon. There are a number of areas where preventive approaches can be applied, including at the level of manufacturing and distribution, regulation, procurement, public health policy and through digital technologies. The following details existing and possible approaches.
Manufacturing and distribution
A critical part of emergency preparedness includes having a sufficient stockpile of essential medicines and medical products that can withstand any sudden shocks or disruptions to production and supply. The advent of the Covid-19 pandemic was a disturbance to both upstream and downstream supply chains. The available stockpile in many countries was quickly exhausted and resupply became a challenge. This led to product shortages and opened up opportunities for FMPs.
Subsequently, there is much to be learned from the Covid-19 pandemic on how to safeguard the manufacturing and distribution of medical products to prevent scarcity. For example, the pandemic has highlighted the vulnerability of global centres of medical product production, such as China and India, to changes in global demand and logistics bottlenecks. This consolidation of manufacturing capacity can result in global shortages when production is reduced or placed under excessive stress.
There is a need to explore internationally-coordinated logistical arrangements to ensure streamlined movement in supply chains that can be employed in the face of an emergency. Complementarily, increasing local or regional capacity to manufacture, even just a fraction of the most critical essential medicines, would help minimise the risk of FMP-related corruption in centralised manufacturing, bolster local economic production and human resource capacity development. Increasing local or regional capacity, however, needs to happen once measures are taken to strengthen regulatory frameworks and minimise corruption risks, such as bribes, submission of fraudulent documentation, intentional use of the wrong APIs or minimum API values, among others. These measures could range from enhancing inspection (including post-marketing surveillance),9cc2c377ac89 investigation, enforcement, and proportionate sanctions and penalties.5d9b8375ae79
In the face of an emergency, new manufacturers emerge to fill gaps in supply. Well-established manufacturers have a higher reputational risk were they to manufacture poor quality products, so targeting oversight or auditing efforts towards new manufacturers and ensuring lists of certified or pre-approved suppliers are made transparent and shared within and between procurement authorities can reduce the possibility of purchases of inferior quality products.
A further preventative measure is the use of internationally-applicable medical product packaging using a centralised track-and-trace system.5ba67713392c Such a system can employ technology that is accessible in countries of all income levels. Nonetheless, the effective implementation of track-and-trace systems depends on contextual factors, such as the willingness to participate by all key stakeholders (including government and supply chain actors), the coordination between them, their knowledge and skills, the regulation and legislation in place, monetary investments, and technical and digital capacities and requirements.8cee1e06e18d
Track-and-trace systems could be piloted as part of the ongoing COVAX Facility. India’s system Co-WIN could serve as inspiration here.0c8ed4600fd0 Doing so would help monitor the distribution of the vaccines and used vials. In addition, a simplified track and trace approach may make it easier for healthcare professionals to monitor for fraudulent copies. Finally, by harmonising packaging using a global standard, the system reduces any added administrative and financial burden of repackaging products at the national level.
Regulation
The majority of NRAs are underfunded and less than 30% across the world have the capacity to carry out the necessary functions to ensure medicines of high quality.f029baf23e8e In 2012, the WHO established the GSMS and its Member State Mechanism (MSM) with the purpose of rallying increased attention and action in the area of SFMPs. GSMS and the MSM have made considerable strides in establishing national focal points and a coordinated alert system, but have experienced chronic underfunding since 2016. There is an urgent need to adequately fund both the coordinating efforts of the WHO and NRAs.
There is also considerable need for increased global regulatory coordination. An example of good practice is the EudraGMDP database, maintained by the EMA. This database consolidates information on the authorisations for manufacturing, import and wholesale-distribution, as well as GDP and GMP and certificates.c100aeda6a2c It includes information from the regulatory authorities from all EU Member States. In 2011, a public version of the database was made available allowing access to information that is not commercially or personally confidential. The purpose of EudraGMDP is to streamline the regulatory efforts of Member States and to protect the medical supply chain by sharing information about, and facilitating the verification of, legitimate suppliers and distributors. EudraGMDP provides a useful blueprint for a global GDP/GMP verification system. In light of the many manufacturers and distributors emerging to aid in the Covid-19 response, a similar global system, even if only specifically for sourcing Covid-related products, would help NRA identify trustworthy partners and alert others to untrustworthy ones.
Stricter regulations placing additional regulatory and quality assurance responsibility on countries of product origin for exports is one possible way to overcome the funding gaps for NRAs in LMICs. The EU, for example, requires GMP certificates from exporting countriesb45346a9e746 and the Nigerian Government enacted a guideline that manufacturers outside Nigeria must file ‘evidence that they are licensed to manufacture drugs for sale (and use) in the country of origin6b1b4bd3a28c
Procurement
Typically, central procurement procedures place less priority on product quality compared to competitive pricing, and while there are examples of FMPs at the same price or higher than quality products, FMPs are often less expensive.1e3b0e5e60e8 This increases the risk that FMPs may be unintentionally procured or that manufacturers, in an effort to maintain their profit margins, intentionally produce substandard products, especially for distribution in countries with limited API or contaminant detection capacity.
‘If you have a price that seems very, very different to others in the marketplace, be that too low or too high, there’s quite often a reason for that. If it is too low, it follows the old adage “if something seems too good to be true, it probably is”’. – Sarah Goldsmith, Crown Agents
In an emergency situation where budgets suffer additional constraints, this risk increases. It is important to remember, medicine of poor or dangerous quality is worse than no medicine at all. Sarah Goldsmith from Crown Agents suggests consolidating purchases as a way to help prevent unintentionally procuring potential SFMPs.
‘A larger quantity is easier to purchase. If you can pool [procurement] and have a larger quantity you are in a better position to buy quality products. Pooling procurement...not only helps in terms of value for money, but also in terms of access to quality products.’
Procurement is a centrepiece in emergency preparedness guidance documents that are now undergoing considerable stress testing in the face of Covid-19. It is important that the implementation of emergency procurement procedures is assessed from the perspective of medical product quality to determine if they are fit for purpose and adapted accordingly.
Finally, coordination between procurement and regulatory authorities is also suggested to increase officials’ understanding of the market and which suppliers are trustworthy.
‘Having a good knowledge of the market out there is particularly helpful and means that you can avoid wasting time with some of the suppliers who have newly come onto the market and may be of dubious quality’ – Sarah Goldsmith
Health policy
Similar to the priority in medical product procurement for low prices, policies that advocate for universal health coverage (UHC) may also wind up unintentionally incentivising minimal costs over quality of products and services.0f2dd039e8a6 Unsurprisingly, manufacturers and distributors are focused on maintaining a healthy bottom line. As a result, UHC policies that facilitate over-prioritisation of low-cost interventions could inadvertently drive manufacturers and distributors to cut corners, leading to poor quality products, especially in jurisdictions that have limited capacity to test the integrity of products. An assessment of existing UHC policies from the perspective of the risks and opportunities for SFMPs is warranted.
A further proposal to reduce the numbers of FMPs circulating in medical supply chains, particularly those that are registered for prevention and treatment of Covid-19, is to include these products in medicines patent pools so that more licensed manufacturers can produce these products in all regions of the world, particularly where manufacturing costs are lower, to increase access for LMICs. Recently, the WHO and its COVAX partners have started collaborating with a South African consortium (Biovac, African Biologics and Vaccines, a network of universities and the African Centres for Disease Control and Prevention) to establish a Covid mRNA vaccine technology transfer hub.4e93564cf51d Whilst these kinds of initiatives are commendable, it is also important to ensure proper oversight and increase efforts to strengthen regulatory measures. Otherwise, corruption risks could easily infiltrate the manufacturing process and threaten any progress on the production of vaccines in developing countries.
At the same time, if the production of Covid-19 products has exclusive patents with licensing at the discretion of individual companies, the likelihood of scarcity increases and this opens up opportunities for FMPs. Such decisions require strong political will and cooperation between governments, international organisations, particularly the World Trade Organization, and private companies, which to date has been gravely lacking. So far companies in Europe and the US have not widely shared their technology and data that would increase production. In Vietnam, however, a biotech company that has had successful vaccine trials and is in the final stages of approval has expressed interest in sharing data and know-how for its locally developed Covid-vaccine with manufacturers in LMICs.e033383bd527
The international nature of medical supply chains necessitates joined-up mechanisms, such as the WHO GSMS and its affiliates, including the Uppsala Monitoring Centre, to address SFMPs. It is important that those few initiatives responsible for medical product quality assurance are appropriately and sustainably funded and empowered. This requires political commitment by national governments and bilateral donor agencies to prioritise addressing SFMPs as part of strengthening overall health systems and health governance portfolios.
Digital technologies
Emerging digital technologies have the potential to transform traditional supply chain models into more efficient and transparent processes with potentially profound impacts on product quality. Many countries acknowledge that the use of emerging digital technologies in addressing SFMPs has considerable potential, but that has yet to be fully realised.
In a review of 60 articles of emerging and existing technologies, Mackey and Nayyar41c30b3b2f98 noted that:
‘Digital solutions are unifying platforms that integrate different types of anti-counterfeiting technologies as complementary solutions, improve information sharing and data collection, and are designed to overcome existing barriers of adoption and implementation. Investment in this next generation technology is essential to ensure the future security and integrity of the global drug supply chain.’
Blockchain is one such technology that has been identified as a possible game changer for medical product quality that can integrate upstream and downstream supply chain procurement, and track and trace products during transportation and distribution to the point of dispensing to end users. In a 2020 UNODC report, the Islamic Development Bank stated that the use of a global blockchain platform for medicines supply chains will ‘coordinate aid delivery and mobilise technical and financial resources…(the) platform enables countries to shop for pre-validated items with verified suppliers with built-in controls to track the procurement process’. This not only helps prevent SFMPs from circulating in supply chains, but also fosters aid effectiveness.
Applying digital solutions should be done with consideration for the need of interoperability, fast transaction processing speed, and high privacy/security needed,e90c9d3c3047 as these may constitute significant challenges, particularly in LMICs. In a 2020 study in Nigeria, Akaba et al reviewed the framework for the adoption of blockchain e-procurement in Nigeria and noted key bottlenecks to include limited political will and financial resources. For LMICs, it may be more appropriate to pilot-test digital technologies with select products, for example vaccines, as reflected in testimony from a Nigerian national regulatory official:
‘I am in support of technology as you need to adapt and update based on global standards. But often initial teething problems can arise...Unless you showcase many examples of successful implementation from abroad and have political support, it can be quite difficult...you need a lot of political will and political support and you need to close your ears (to criticism) …if the political leaders are motivated it may work.’
Detection
Where it is not possible to prevent the manufacturing and distribution of FMPs, the next stronghold is to detect these products in the supply chain, ideally before they reach end users, or through monitoring their effects on patients through adverse events or treatment failure. The following details detection strategies that build upon existing national and global detection infrastructure.
Detection methods
There exist myriad screening devices to test the quality of a medical product. According to a review of 41 devices conducted by Vickers et al,93e5abd5c8d7 the most accurate screening devices are both expensive and require special training to operate. Screening devices that are portable and intended for use in low resource settings, while often able to identify if there is no or very little API, are inadequate to distinguish a suboptimal level of API or assess the dissolution of a product. Suspicious products must often be sent for additional confirmatory testing at centralised labs. This can lead to delays in detection, as well as the quarantining and removal of products from circulation. This is particularly concerning as according to a comparative meta-analysis by McManus and Naughton,e80a6a0b03e8 over a six-year period there was a considerable decrease in studies that detected products containing no API (47% in 2013; 18% in 2019), while the number of studies that identified products with an inadequate amount of API remained practically unchanged (93% in 2013; 94% in 2019). This suggests that there is a need for improved field-ready screening methods, as well as improved laboratory capacity, and increased testing comparability through standardised testing protocols to adequately respond to these developments.
One method to overcome this hurdle in the meantime would be to develop a flexible surveillance protocol to prioritise drug categories that are most at risk of being falsified. At-risk products can be forecasted by consulting reports of epidemiological trends or past FMPs identified that include information about targeted product categories to develop a list of ‘high risk products’.
Reporting mechanisms
There is a need to break down existing silos and localised regulatory and oversight capacity through global mechanisms that collect and process reports of poor quality or harmful medical products in supply chains, such as the WHO GSMS and Uppsala Monitoring Centre. There is also the need for greater cooperation of NRAs and RRAs, as well as knowledge and technology transfer to NRAs with limited capacity in order to strengthen their detection and reporting capabilities, especially in those jurisdictions that are often affected by SFMPs.
Frontline healthcare professionals are in a unique position to identify and report products of suspicious quality. However, only 12% of all cases reported to the WHO GSMS are initiated by healthcare professionals.c08699089e68 Efforts should be made to provide additional training on how to detect and report in order to increase the number of healthcare professionals reporting. To reduce any fear of reprisal or danger to self, reporting mechanisms must ensure protection of healthcare professionals, for example through digital, anonymous reporting platforms.
There already exist healthcare professional training materials for SFMP detection, such as the US FDA Supply Chain Security Tool Kit, developed together with Asia-Pacific Economic Cooperation countries.eb128efaf124 It spans the entire supply chain, providing training on processes, procedures, and tools directed at enhancing global medical product quality and security across ten distinct categories, including: GDP/GMP, track and trace, digital monitoring and online pharmacy.
Public awareness raising
In addition to training healthcare professionals, procurement officials and regulators, increasing public awareness to identify FMPs, or increasing the digital literacy of key populations that rely on or are likely to turn to online sale of medical products, can help increase detection rates. For example, a case of falsified medicines sales in Ontario, Canada, was uncovered by a patient who recognised that the blood pressure medication from a registered pharmacy ‘did not look right’. This kicked off an investigation that later led to further schemes being uncovered in nearby areas.dcdadecb4959 In the context of Covid-19, media campaigns can provide the public with accurate and reliable information about at-risk medical products to facilitate awareness raising and public reporting.
Artificial intelligence and other technologies
Artificial intelligence (AI) can support the detection of FMP sales, track their distribution, dispensing and consumption, and compare relevant data on product origin and journey in supply chains with adverse events.a334c5966c56 This is particularly relevant for Covid-related products, as well as those products that may be impacted by supply chain disruptions caused by the pandemic.
Use of AI and other digital technologies for detection should be approached with caution. It should take into consideration the costs, the feasibility of integration, application ownership and sustainability, and balance the thrill of new technology with the need to scale-up or increase the user-friendliness of effective, existing interventions, like the use of SMS. In addition, there are several ethical challenges involved in the development of AI-driven systems, including privacy concerns, surveillance issues, and opportunities for opaque decision-making processes.57f29e8028de
Response
Responding to FMPs is the last line of defence against poor quality and even harmful products in medical supply chains. The bulk of FMP responses lie in improving medical supply chain governance. This includes strengthening NRA capacity and international mechanisms in order for them to carry out their functions following the detection of FMPs, such as quarantining and recalling products, and ensuring robust and proportional legal consequences for those involved in the manufacture, distribution and sale of FMPs.
Regulatory resourcing
Part of NRA responsibilities is the safe removal of poor-quality products from circulation in supply chains when they are identified. As stated above, less than 30% of NRAs worldwide have the capacity to carry out the necessary functions in order to guarantee patients’ access to medicines, vaccines and other products that do not cause them harm.04d4118aecd0 This is at least in part due to under-resourcing of regulatory authorities and other oversight bodies; both financial, human and technical resources are needed. Additionally, support for inter-authority cooperation is critical to prevent cross-border entry of FMPs, particularly between the export and import locations.
Legal frameworks
There exist several underutilised legal guidance documents and conventions for SFMPs that should be used to inform and adapt national criminal legislation. One example of this is the MEDICRIME Convention. This is a legally-binding international criminal law put forth by the Council of Europe to address SFMPs. It sets out legal mechanisms and precedence for adaptation into national law. The MEDICRIME Convention was opened for signature in 2011, and to this date it has only been ratified by 18 countries and signed by 15 others. Only four countries that are not members of the Council of Europe have ratified it; 22 countries that are members of the Council of Europe have yet to sign it.1c1e33b8fe17 This is a lost opportunity, as most states are unable to adequately prosecute medical product-related crimes, or their existing legal frameworks are woefully inadequate. Currently, the sanctions for manufacture, distribution and sale of illicit drugs carries a substantially higher penalty than SFMP-related crimes, although it is argued that the impact of SFMPs has further-reaching, negative implications.3922912ead31 The relative impunity of SFMP-related crime makes it an attractive area of criminal activity.
The UNODCe7cdad2a7969 has also issued guidance for addressing SFMP-related crime. This document provides member states with guidance on good legislative practices, including examples of legislature that can be integrated into national law.
Conclusion and recommendations
This U4 Issue set out to understand the role and extent of corruption in FMPs, determine the unique challenges presented by the Covid-19 pandemic, particularly in LMICs, and present possible solutions and approaches to address FMP-related corruption.
Based on the findings, there are many drivers of poor medical product quality, including novel disruptions such as the Covid-19 pandemic. Other drivers include, for example, excessive product shortages either through real or created scarcity, desperation and anxiety among the public, high costs of quality medical products, and most consistently, limited regulatory and political capacity to improve the oversight and governance of medical products.
In summary, FMP-related corruption manifests in five major ways:
- In legitimate manufacturing and distribution, in the form of fraudulent GMP/GDP certification, intentional GMP/GDP non-compliance, and outright interception of products in transit which may be the result of collusion.
- At the regulatory level, whereby regulatory officials may collude with manufacturers and distributors of FMPs by providing unwarranted GMP/GDP certification or product approval. Regulatory authorities may also delay approval of competitor products due to conflicts of interest or collusion with FMP suppliers.
- In procurement systems that lack transparency of specifications, prices or awardees, procurement officials may solicit bribes from and collude with manufacturers and suppliers of FMPs in order to participate in procurement tenders. Procurement procedures that prioritise low prices may inadvertently increase the risk of FMPs.
- At the level of district or central government, officials can engage in corrupt schemes through personal affiliations or interests in companies’ manufacturing, distributing or selling FMPs. Political leaders may prioritise themselves, their families, friends and allies for new treatments or vaccines, which in turn increases product scarcity and opens up new opportunities for FMPs to be introduced in markets. Additionally, those in power may intentionally fail to appropriately fund NRAs because of conflicts of interests with manufacturers and distributors of FMPs.
- Manufacturers and distributors can bribe healthcare professionals to prescribe their products, especially in contexts where salaries are low or not consistently paid. Healthcare professionals may themselves compromise product quality to increase their own profits. Finally, healthcare professional discretion may lead them to exercise nepotism and favouritism in dispensing legitimate products, contributing to product scarcity and increasing opportunities for FMPs.
Based on the findings of this U4 Issue, the following recommendations are made for governments, their regulatory authorities, and development actors:
FMP-related corruption prevention strategies for pandemic preparedness
Covid-19 provides real-time opportunities to identify gaps in broader supply chain protections against FMPs and to test technologies and innovations that can prevent and detect FMPs and FMP-related corruption. All of these can help establish proof of concept, inform pandemic preparedness guidance, and contribute to overall supply chain improvements.
Pandemic preparedness guidance should be reviewed through the lens of FMPs, particularly regarding how supply chain disruption, weak regulatory capacity, expedited procedural requirements such as for procurement, and societal dynamics like desperation, anxiety, and misinformation contribute to FMPs.
Similarly, health innovations, such as treatments, vaccines and technologies developed to respond to a health emergency must be accompanied by proactive strategies for preventing FMPs and FMP-related corruption to ensure procurement integrity.
Governments, bilateral agencies and international NGOs should use the unique circumstances of the Covid-19 pandemic to carry out supply chain analyses for FMP-related risks, including corruption risks. By surveying the holes in supply chains that can facilitate FMPs, what is needed to fill such holes can be established to inform pandemic-preparedness guidance.
The US Pharmacopeia32d568f3d2c0 has outlined specific recommendations for regulatory authorities to reduce the risk of FMPs’ proliferation in low and middle-income countries in pandemic scenarios. These include: (i) sharing product quality surveillance information with other regulatory agencies, such as inspection reports, product quality test results, and other data relevant to medical product quality; (ii) implementing risk-based quality surveillance, including post-marketing quality surveillance; and (iii) making use of tech-based strategies to remotely monitor medical product quality, such as social media analyses for insights into product quality, patient reporting, serialisation, and track and trace technologies.
Strengthen cross-border counter-FMP coordination
It is necessary to break down silos in regulatory and oversight capacity that share the common goals to prevent, detect and respond to FMPs in supply chains. One obvious approach can be to facilitate knowledge and technology transfer between NRAs through North-South, South-South and Triangular cooperation to strengthen prevention and detection capacities and foster mutual learning. A case in point is the cooperation between Brazilian and Cape Verdean regulatory authorities. From 2011 to 2020, Brazil provided capacity and technical assistance to support the institutional strengthening of the agency for Regulation and Supervision of Pharmaceutical and Food Products in Cabo Verde.c952a77ac9c7 Establishing an understanding of the strengths and weaknesses of cross-border coordination mechanisms should be included as part of an assessment to identify gaps and challenges to ensuring medical product quality.
Another area that would benefit from multistakeholder coordination is procurement, especially when this has been done with donor funding. Experience shows that donors tend to have different quality assurance policies, making it challenging for country recipients to comply with all procurement regulations, assure the quality of medical products, and reduce corruption risks. In 2012, the World Bank published a report outlining pragmatic approaches to address these issues. The report showcased the discussions of a meeting hosted by the WHO and Global Fund and attended by 33 participants from 17 organisations, mostly from the donor community. Amongst its recommendations were to ‘continue developing a risk-based categorisation of essential medicines; harmonise tools to assess procurement agencies’ capacities and risks; harmonise quality assurance approaches; and develop a mechanism to share information’.722c5367fae1
Regulatory financing and expansion
The limited technical and financial capacity of regulatory agencies needs to be urgently addressed. The majority of national and regional regulatory authorities are either underfunded or rely heavily on private sector fees, which can undermine their integrity. Public and donor funds need to be increased and sustainably allocated to support these agencies in order to strengthen global protections against FMPs.
This is particularly true for the online pharmacy space, which is at present under-regulated and a serious risk for FMPs reaching end users. Medical product regulators need to have a seat at the table where legal frameworks for the regulation of the Internet are decided. The International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) and the International Pharmaceutical Federation (FIP) have called on governments, international organisations and the private sector to redress the online sale of falsified medicines, and especially advocated for stronger regulations, including tougher sanctions and more effective enforcement measures.a84a35a8e2ee
Similarly, there is a need to expand regulatory oversight to unregulated markets that commonly operate in many LMICs. Unregulated black or grey markets offer a platform for the distribution and sale of FMPs. Often these types of markets serve an important function in healthcare systems, so it is not simply a matter of dissolving these types of markets, but rather improving regulation and integrating oversight of their activities into the remit of regulatory authorities. In times of health crises, the hoarding of medicines and medical products is also likely to happen, leading to an increase in panic buying in the black market and the proliferation of FMPs. Supervising the production and circulation chain is therefore key to reduce any profiteering by manufacturers, importers, and retailers. Governments can set up task forces or committees at the state and/or national level to strictly monitor the circulation of medical supplies, as has happened in the Indian state of Odisha.0f1079ae73e3
Integrating FMP research and innovation
To the authors’ knowledge, this is one of the first publications to look in depth at how corruption and corruption risk facilitates FMPs in supply chains. This is congruent with a general dearth of data on SFMPs, a difficult topic to research due to the involvement of ruthless and unscrupulous criminal groups that could pose serious dangers for primary researchers. Accordingly, governments and development actors involved in supply chain integrity should sustainably support research into FMPs and the role of corruption in order to better understand their prevalence across geographies and income levels.
Such research should include identifying ways to improve the quality and standardisation of available testing for suspected SFMPs. Where increases in laboratory testing capacity is targeted or desired, sustainable financing for the supply of quality testing reagents, which are often overlooked, must also be provided, especially in the current context of the Covid-19 pandemic.
Research conducted should be used to inform the feasible adoption of emerging technologies like blockchain and AI that documents promising ways to improve supply chain oversight of product quality. Where possible, innovative strategies to address FMPs should seek to scale existing, effective low-tech interventions.
Reform legal frameworks and FMP facilitative policies
A general lack of adequately punitive legislation to deter the manufacturing, distribution and sale of SFMPs by criminals and corrupt actors represents a lost opportunity to deter FMP activity. There is a particular need to increase the weight of legal penalties to ensure they provide sufficient deterrence proportionate to the rewards of manufacturing and selling FMPs. It is also necessary to ensure that national and regional legislation is aligned with the need for cross-border and international participation, as well as emerging technological developments for improving medicines regulation and product quality.
Governments are advised to carry out policy analyses against established good practice legislation and existing criminal law conventions, such as the MEDICRIME Convention, to identify and fill gaps. Joining and ratifying the MEDICRIME Convention can also help enhance countries’ regulatory frameworks against FMPs. The MEDICRIME Convention ‘makes falsification a criminal offence; fosters effective prosecutions; seizes proceeds from falsification; and ensures national and international cooperation to fight this crime’.70c5834a7566
Similarly, governments, with support from development actors where relevant, should carry out assessments of existing public health policy that may place too strong of an emphasis on price cutting and lowering health expenditure, as this can contribute to the manufacture and circulation of FMPs in supply chains. For example, policies promoting UHC that may prioritise price over quality or national objectives to promote local manufacturing without sufficient quality assurance mechanisms may inadvertently lead to procurement and provision of poor-quality medical products.
Development actors that promote the lowering of health expenditures should review their development policies to ensure that they are not inadvertently pushing manufacturers to produce poor quality products or to create opportunities for lower cost, falsified products to enter supply chains.
SFMP awareness raising and training
Increasing awareness and improving systems for reporting SFMPs by healthcare professionals and the general public, including training to be able to identify SFMPs, could be an effective way to create a last line of defence and prevent SFMPs from reaching end users. Public awareness efforts should particularly target Internet literacy to protect against online supply of FMPs. Governments and the donor community should support initiatives, such as Fight the Fakes, a multi-stakeholder campaign that raises awareness about the negative impacts of falsified medicines. It also provides a space for victims to share their stories and for those working to reduce FMP proliferation.cf67a887b083
Health professionals should have access to regular training and briefs regarding vulnerable and suspect products. Reporting mechanisms must be user-friendly and allow for anonymous reporting that protects whistle-blowers, particularly as FMPs can have connections to criminal syndicates. Where appropriate, providing incentives for reporting should be tested.
Specific recommendations for donor agencies
- Promote and support fraud-risk assessments and appropriate mitigation strategies to strengthen the supply chains of medical products. This will not only uncover areas where corruption is likely to arise, but will also identify other risks that could hinder the supply of safe and quality medical products. Improving the efficiency of supply chains through risk assessments can contribute to making these processes more transparent, effective, and accountable.
- Fund studies on how to contain the proliferation of falsified medicines through e-commerce, and how to improve international coordination of logistical arrangements to ensure streamlined movement through supply chains. These studies may focus on assessing current approaches and/or evaluating the benefits of using technologies such as track-and-trace systems, blockchain and artificial intelligence, among others.
- Fund further studies that assess the role of corruption in the proliferation of FMPs to better understand their prevalence across geographies and income levels.
- Prioritise addressing SFMPs as part of strengthening overall health systems and health governance portfolios. For example, when importing essential medicines and/or medical products as part of their health programmatic work, donor agencies should support national medical product and drug supply agencies to conduct due diligence protocols and guarantee that they only work with well-established manufacturers, engage in pooled procurement when possible, and focus on strengthening their measures to inspect and investigate potential sources of corruption within their supply chains.
- Donor governments should also cooperate with governments by providing them with information about any irregularities they witness and offer policy and technical support to governments to enhance their enforcement measures. Donors can support the strengthening of national and local regulatory agencies, since most of them are either underfunded or rely heavily on private sector fees, which can undermine their integrity.
- Encourage North-South, South-South, and Triangular Cooperation amongst different regulatory agencies with respect to how best to tackle corruption in supply chains and the proliferation of falsified medicines.
- Donors can use their leverage in partner countries to promote countries’ ratification of the MEDICRIME Convention.
- Increase funding for the WHO GSMS and its Member State Mechanism. So far, these mechanisms have succeeded in rallying attention and action on SFMPs. They have made considerable strides in establishing national focal points and a coordinated alert system. Nonetheless, they are chronically underfunded.
- Contribute to the development of a global database, similar to EudraGMDP database, either through allocating funds to it or by leading on its development. Such a database could consolidate global information on authorisations for manufacturing, import and whole-sale distribution, as well as GDP and GMP certificates. By making this information publicly available, this database would help streamline regulatory efforts from different NRAs and verify the legitimacy of suppliers and distributors. Since this is quite a big undertaking, the development of such a global database might only focus on Covid-19 related products as a starting point.
- Emphasise on the need for quality medicines and medical products when advocating for UHC. Donor governments should also support countries through capacity-building initiatives that help assess how their existing UHC limits and/or enables the proliferation of SFMPs.
- Actively engage in the development of a flexible surveillance protocol to prioritise drug categories that are most at risk of being falsified.
- Collaborate with other donor agencies to harmonise tools to assess procurement agencies’ capacities and risks, harmonise quality assurance approaches, and develop a mechanism to share information.
- Provide financial and technical support in the delivery of training initiatives that better equip frontline healthcare workers as well as patients to detect and report SFMPs. Equally, support initiatives that seek to raise public awareness about the negative impacts of falsified medicines.
- UNGA, 2009.
- Behner, Hecht and Wahl, 2017.
- WHO, 2017a.
- IOM, 2013.
- The 11 product categories listed by the GSMS in their 2017 report include anaesthetics and painkillers, antibiotics, cancer medicines, contraception and fertility treatments, diabetes medicines, heart medicines, HIV/hepatitis medicines, lifestyle products (including those for cosmetic use, erectile dysfunction, body-building and dieting), malaria medicines, mental health medicines, and vaccines.
- WHO, 2017a.
- Ozawa et al., 2018.
- WHO, 2016.
- WHO, 2017c.
- 2017.
- WHO, 2017c.
- Note that the IDDO Medicines Quality Monitoring Globe Index records all publicly available news reports of confirmed and suspected substandard and falsified medical products. It does not filter out duplicate reports of a singular event.
- INTERPOL, 2020.
- IDDO, 2021.
- Jones, 2020; Otieno, 2020; Soylu, 2020; UN News, 2020.
- INTERPOL, 2021a.
- Ukpe, 2020.
- Pee et al., 2021.
- UNODC, 2020a; UNODC, 2020b.
- Tsertsvadze et al, 2015.
- NIH, 2020.
- Newton and Bond, 2019.
- Besson, 2020.
- Long, 2021.
- 2019.
- Collie, 2020; Swissmedic, 2020; Szucs, 2020.
- Hope, 2020.
- Ngamkam, 2020.
- El Shamaa, 2020.
- Europol, 2020.
- US FDA, 2021.
- Tull, 2018.
- Luhana, 2020.
- Fröhlich et al, 2020; Lapid, 2020.
- Mackey et al., 2020.
- Piranty, 2020.
- Bate, 2020.
- Guerin et al., 2020; Kindzeka, 2020; Piranty, 2020; WHO, 2020b.
- WHO, 2020b.
- WHO, 2017b.
- Roth et al, 2018.
- WHO, 2017c.
- Pisani et al, 2019.
- Ibid.
- According to the definitions used by the European Union, biocidal products refer to those products used to control unwanted organisms that are harmful to human or animal health or to the environment, or that cause damage to human activities. The term cosmetic products refers to a range of products from everyday hygiene products such as soap, shampoo, deodorant, and toothpaste to luxury beauty items including perfumes and makeup.
- Berardi et al, 2020.
- Economic Times, 2020; NHS Digital, 2020; Rubin, 2020.
- EMA, 2020a; MHRA, 2021.
- 2020.
- 2018.
- Newton and Bond, 2019.
- Kovacs et al., 2014.
- Vickers et al., 2018.
- Economic Times, 2020; NHS Digital, 2020; Rubin, 2020.
- Gabay, 2015.
- INTERPOL, 2021a.
- Good Manufacturing Practices describe the minimum standard that a medicines manufacturer must meet in their production processes.
- Good Distribution Practices describe the minimum standards that a wholesale distributor must meet to ensure that the quality and integrity of medicines is maintained throughout the supply chain.
- Pisani et al, 2019.
- 2019.
- Ibid.
- Ibid.
- OECD, 2020.
- European Commission, 2018.
- 2002.
- Newton and Bond, 2020; Tesfaye et al, 2020; WHO, 2017b.
- Behner, Hecht and Wahl, 2017.
- Vickers et al, 2018.
- WHO, 2017c.
- b.
- UNICEF, 2021.
- Davies and Furneaux, 2021.
- Federal Ministry of Health, 2021.
- BBC, 2021.
- Cabato, 2021.
- INTERPOL, 2021b.
- The Star, 2021.
- Jercich, 2021.
- WHO, 2017b.
- Nakkazi, 2021.
- 2020.
- Liu, 2018.
- Chartterjee, 2020.
- IWPR, 2017.
- Emmanuel, 2021.
- Aljazeera, 2021.
- EMA, 2021b.
- Dou, 2020.
- Bergonia, 2020.
- Dou, 2020.
- Ibid.
- Mackey et al., 2020.
- EMA, 2020.
- Transparency International, 2016.
- Nyambe, 2021.
- Pisani et al, 2019.
- 2021.
- OCCRP, 2021.
- The Observer, 2020.
- Taylor, 2021.
- Heath, 2021.
- Kenyon, 2021.
- Khan, 2012.
- Rasheed et al., 2019; TI Pakistan, 2020.
- BBC, 2021.
- Pauls, 2021.
- 2017.
- Gupta & Yeung, 2021.
- Deutsche Welle, 2018.
- Tucker, 2021.
- De Freitas, 2021.
- Cepeda, 2021.
- Post-marketing surveillance is comprised of “surveillance activities that occur following market approval of a medicine, including maintenance of product authorisation and/or registration of variations or renewals; regular inspections of manufacturers, wholesalers, distributors, and retailers; quality control testing; pharmacovigilance; promotion control; public reporting of poor-quality products; handling of market complaints; and removal and disposal of non-compliant products.” (USAID and USP, 2018).
- OECD, 2020.
- WHO, n.d.
- Kootstra and Kleinhout-Vliek, 2021.
- Court, 2021.
- WHO, 2018.
- EMA, 2021c.
- Pisani et al, 2019.
- NAFDAC, 2018.
- Pisani et al, 2019.
- Pisani et al, 2019.
- WHO, 2021.
- Ravelo, 2021.
- 2017.
- Ahmad, 2020.
- 2018.
- 2020.
- WHO, 2017c.
- FDA-APEC, 2020.
- CBC, 2005; Köhler, 2005.
- Mackey, 2020.
- Aarvik, 2019.
- WHO, 2018.
- Council of Europe, 2021.
- Di Giorgio and Russo, 2019.
- 2019.
- USP, 2021.
- South-South Galaxy, n.d.
- Moore et. al., 2012, p. xii.
- IFPMA, n.d.
- Verma, 2021.
- IFPMA, n.d.
- IFMPA, n.d.
