1. Introduction
Covid-19 has been an unprecedented challenge for global public health, good governance, and social and economic development. In May 2020 a historic resolution of the World Health Assembly called on all parties to guarantee universal access to Covid-19 vaccines as a way out of the pandemic. Member states agreed to support negotiations for an international treaty on pandemics that would guarantee ‘universal and equitable access to medical solutions, such as vaccines, medicines and diagnostics.’ In March 2021, more than 175 member states of the United Nations (UN) supported the Political Declaration on Equitable Global Access to COVID-19 Vaccines, and the UN Human Rights Council adopted a resolution calling on ‘States and other relevant stakeholders to take appropriate measures to guarantee the fair, transparent, equitable, efficient, universal and timely access and distribution’ of these vaccines. Universal and equitable access to Covid-19 vaccines is therefore a global commitment, a human rights obligation, and, above all, a central pillar of the global pandemic response.
The development of effective vaccines at record speed has been a remarkable success. However, their distribution, according to UN Secretary-General António Guterres, has been ‘wildly uneven and unfair’, despite global mechanisms that are supposed to guarantee universal access. Low- and middle-income countries have the least access to Covid-19 vaccines. COVAX was set up as a multilateral initiative under the World Health Organization; Gavi, the Vaccine Alliance; and the Coalition for Epidemic Preparedness Innovations (CEPI). It is a global mechanism for pooled procurement to ensure fair and equitable access to Covid-19 vaccines. As of 29 September 2021, however, COVAX has been able to channel just 311 million doses to some 143 countries in need. This is a tiny fraction of the 6.1 billion vaccine doses that have been delivered around the globe, mostly to high-income countries.
The mismatch between enormous vaccine demand and limited supply has been called a ‘catastrophic moral failure’ and an ‘infringement upon human rights’. As the wait for vaccines continues for many around the world, people are dying and millions of lives are at risk, with low-income and other disadvantaged groups disproportionately affected. Livelihoods and educational equality are suffering; violence is on the rise; and migration is expected to increase.e44fd8a2426c Compared to wealthy countries, low-income countries will take far longer to recover from the pandemic and will see greater social unrest along the way.
Transparency and accountability in the Covid-19 vaccine response have so far been very weak.a896b62a5781 In most countries there is a lack of public information about the terms and conditions that underlie public funding for vaccine development as well as bilateral technology transfers, voluntary licences, and contract manufacturing agreements. This hinders informed discussions about options for scaling up vaccine production, makes it difficult to hold principal actors accountable, and prevents fair and equitable vaccine distribution.
A root cause is the power and information asymmetries between a ‘historically secretive’6a3f7b4b0efb pharmaceutical industry, on one hand, and national governments and multilateral organisations, on the other. These asymmetries have negative repercussions at critical points throughout the Covid-19 vaccine value chain. Opacity and secrecy in vaccine contracts, including those of COVAX, have been widely justified as necessary to protect proprietary information and permit future deals with pharmaceutical companies. Indeed, drug procurement contracts have rarely been disclosed to the public, even before the pandemic.
However, given the extraordinary amount of public funding channelled to the development of Covid-19 vaccines, this culture of non-transparency has become unreasonable. It has resulted in poor countries paying more than rich countries for the same vaccine. It has also contributed to uncertain vaccine delivery schedules and made it difficult to conduct public monitoring and hold pharmaceutical companies accountable when they fail to meet their commitments. Secrecy and opacity obscure a global overview of how many vaccines are and will be available to which country and by when.
There is also insufficient public information on bilateral donations by vaccine-producing countries to vaccine-recipient countries. Bilateral donations are often allocated strategically, to serve the interests of the donor country, rather than based on a global equity framework such as the COVAX scheme. Consequently, some bilateral initiatives are seen as a form of ‘vaccine diplomacy’,3323e00f1f9b while others are portrayed as either charity or solidarity. These two-way transactions have undermined COVAX as the global mechanism to ensure fair and equitable access to vaccines.
Lack of transparency in clinical trials has fuelled vaccination hesitancy, threatening progress towards herd immunity. Finally, in many countries, national plans for the Covid-19 vaccine roll-out have been opaque, and several corruption scandals involving preferential vaccine access have undermined the legitimacy of the Covid response in general.5a01a37d4722
Lifting these many veils of opacity is crucial to ensure access to vaccines for all. Viral diseases do not recognise borders. The COVAX website declares, ‘With a fast-moving pandemic, no one is safe unless everyone is safe.’ Urgent action to ensure equitable vaccine access worldwide is not only a moral imperative, to guarantee the human right to health for all, but also serves the self-interest of every country, rich and poor alike. This requires improving transparency in key areas of the Covid-19 vaccine value chain: in development, production, distribution, and contracting. Towards that end, this U4 Issue provides recommendations for donor agencies, governments, multilateral organisations, and civil society. In the short to medium term, an international vaccine transparency initiative should be developed to mitigate current mistakes and shortcomings and strengthen the world’s Covid-19 response. In the long term, the initiative should also build on lessons learned with a view to ensuring a more effective global response to future pandemics.
2. From opacity to transparency in the Covid-19 vaccine value chain
Transparency is a key principle of good governance, and access to information is a fundamental right anchored in the freedom of information laws of 119 countries.Both are needed to ensure accountability for public decision making as well as performance in the Covid-19 vaccine response. Understanding how decisions are made requires access to information about the procedures and criteria used, while understanding why decisions are made requires disclosure of the arguments used by the different actors for or against different policy options.
Transparency and accountability go hand in hand. This U4 Issue focuses on the former: on transparency and access to information and its crucial role in ensuring fair and equitable access to Covid-19 vaccines. Future studies should review the accountability mechanisms in place to assess the performance of the different actors involved in the global vaccine response.
At the national level, various sources have published insights and guidance on promoting transparency and accountability in the Covid-19 response.ef04516ac2fc Less attention has been paid to how opacity and secrecy at the international level influence vaccine access. This paper attempts to fill this gap by examining the international dimensions of the Covid-19 vaccine value chain, namely research and development, manufacturing, international distribution, and contracting (Figure 1). These international stages precede delivery of the final product to countries for roll-out of their national vaccination campaigns.
The remainder of section 2 looks at each of these stages in turn. For each, we consider the current situation and dynamics at play, explaining why transparency and access to information is crucial, and suggest what should be done to remedy current unsatisfactory practices. Specific recommendations for donor agencies and international organisations are presented in section 3.
Figure 1. International dimensions of the Covid-19 vaccine value chain
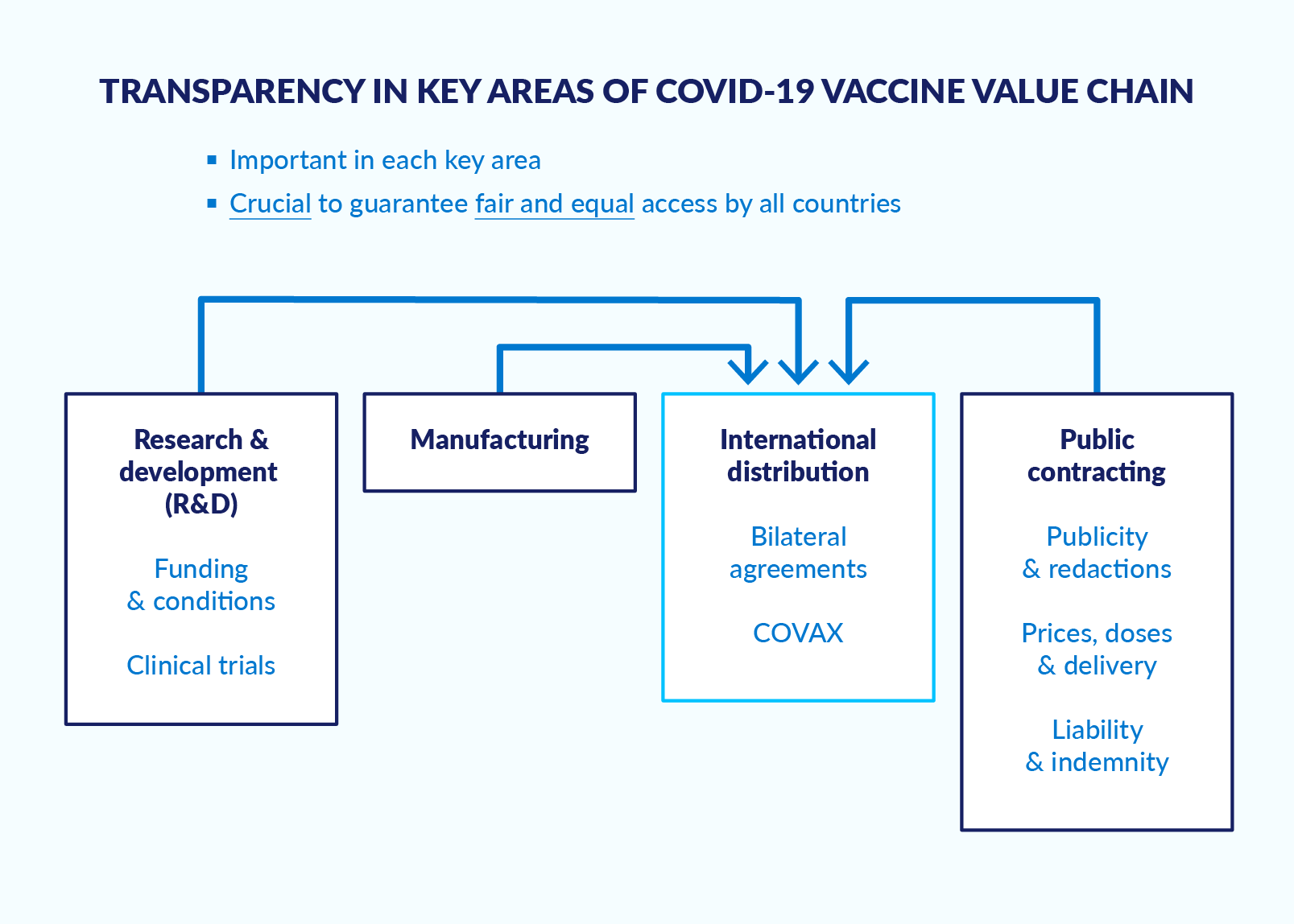
Source: Author.
2.1 Research and development
The research and development (R&D) pillar of the vaccine value chain covers a wide range of processes. We focus on public funding for vaccine discovery and development and on clinical trials.
2.1.1 The current situation in R&D
Since the Covid-19 outbreak started, unprecedented amounts of public funding have been made available for Covid-19 vaccine development, and several different vaccines have been developed at record speed. Mechanisms have included grants to universities, credits with risk-sharing arrangements to biotech companies, and advance purchase agreements (APAs) with pharmaceutical companies, among others. Nonetheless, there is a lack of easily accessible public information on which entities have received funding, in what amounts, through what mechanisms, and under what conditions. Universities, international civil society organisations, and the media have attempted to track the funding for the different vaccine candidates, but the overall picture remains incomplete and sometimes confusing.
According to the Knowledge Network on Innovation and Access to Medicines, based at the Global Health Centre in Geneva, the public sector and CEPI have ‘invested USD 5.6 billion in developing Covid-19 vaccines, and more than USD 51.1 billion when considering APAs as an incentive for development.’18abb2bade5d The United States and Germany are by far the biggest funders, followed by the United Kingdom (UK), the European Union (EU), Canada, Norway, Singapore, China, and Saudi Arabia. The kENUP Foundation estimates that by January 2021 the public sector had provided about 88.3 billion euros for Covid-19 vaccine development. Most of these funds were spent through advance purchase agreements, with 7% channelled through loans or grants.d4b3176b2d5f
This shows that public funding through APAs by governments, multilateral organisations, and multilateral mechanisms such as COVAX (CEPI) has played a crucial role in financing Covid-19 vaccine R&D. APAs considerably lower the risks that vaccine developers assume when they embark on R&D and scale up vaccine production, but little attention has been paid to the need for public information about these agreements. Their terms and conditions vary,1f11910c84f3 but all contain crucial information about the number of doses, prices, delivery schedules, liabilities, and more. Public access to this information would enable everyone to know, for example, who is securing deals with whom, and whether high-income countries that invest large amounts of public funds in APAs receive discounts on price. This could help explain why countries and multilateral organisations frequently pay different prices for the same vaccine (see section 2.4).
In addition, public access to information on the terms of Covid-19 R&D grants, loans, and credits would enable scrutiny on how taxpayer money is spent. For example, in the early days of its Covid-19 vaccine research, Oxford University stated that it would provide the vaccine ‘recipe’ as a public good, so that the drug could be produced around the world. However, shortly afterwards, Oxford University pursued a commercial model for vaccine production with AstraZeneca. Questions remain as to why the initial commitment to openly share the vaccine recipe was overturned, and why this decision, which has certainly contributed to today’s undersupply of vaccines, did not generate public debate.
The Oxford/AstraZeneca case also invites us to question the role public funding should play in the development of vaccines for health emergencies. Should developers receiving public funding be required to share the vaccine recipe, if successful? What would be the conditions for a transparent collective-action approach among countries and companies? And finally, what are the lessons learned for future pandemics?dea9ddeeb216
Finally, public funding for Covid-19 vaccine R&D is also channelled through CEPI, a global partnership launched in 2017 to fund the development of vaccines to stop future epidemics. CEPI is a partner of the international COVAX facility. As of January 2021, public funding to CEPI for Covid-19 vaccines amounted to US$ 1.4 billion, and CEPI had provided grants to 10 different developers with provisions to ensure equitable access to the vaccines. However, CEPI has been criticised for being ‘too secretive about the conditions in its contracts and for not pushing vaccine developers harder to achieve better terms.’ CEPI began publishing some of these details after the US-based organisation Public Citizen released a report questioning CEPI’s equitable access provisions in its agreements with vaccine developers. However, vital information is still being withheld.4381793cfbea
Specific data on investments made by pharmaceutical companies into Covid-19 vaccines R&D has not been disclosed. As a result, the size of total investment, both public and private, is not known. But it is clear that public funders of vaccine R&D missed the opportunity to define key parameters for the expected race for vaccine development and in that way ensure sufficient vaccine supply worldwide.
Another crucial area for transparency relates to the clinical trials of Covid-19 vaccine candidates. While clinical trial transparency is a challenge for vaccine development even under normal circumstances, the unprecedented speed at which Covid-19 vaccine trials were undertaken has exacerbated these problems.7b3eadd39059 A recent Transparency International Global Health report examined 86 registered clinical trials of 20 vaccine candidates and found that full clinical study reports are likely to be made available in only two of the nine jurisdictions where vaccine developers are based. The study also found that clinical trial protocols were available for only 10 of the 86 trials examined. Furthermore, reports often presented high-level rather than full results, and this was often done via press release rather than through a peer-review process. Obscuring the situation even more, some Latin American countries, for example, entered into confidential agreements with pharmaceutical firms for the conduct of clinical trials without publicly available information on the objectives, justification, or implications of these confidentiality clauses.6b944a4b44ef
These problems, according to Transparency International, arise from an ‘incoherent global clinical trial transparency policy landscape’, and the outcome is a ‘selective sharing of results and a failure to explain methodological details that are key to interpreting the results.’436fd3a6ed4a Lack of public access to information about trials has contributed to public distrust in the Covid-19 vaccines and has furthered market distortions in the already oligopolistic vaccine market.b726066a2aa7
2.1.2 R&D: What should be done?
Public information about public funding for R&D of Covid-19 vaccines is essential for a number of reasons. First, the unprecedented quantity of public funds for vaccine R&D provided through grants, credits, and APAs, among others, provides a powerful justification for calls for temporary waivers of patents, voluntary technology transfer, and/or compulsory licensing to scale up vaccine manufacturing and guarantee fair access for all.58aad5f29471
Second, access to information about the conditions of public funding for Covid-19 vaccine development would help the public understand whether funding parties, whether governments or COVAX through CEPI, have access to preferential delivery commitments and/or special prices. This could provide a basis for measures to counterbalance the resulting foreseeable inequities in distribution.
Finally, clinical trial transparency is crucial to prevent the manipulation or distortion of data, to allow for independent review of potential benefits and risks of the vaccines, to prevent manipulation of markets, and to enable public scrutiny of health regulatory agencies.356d7cbf976b Most importantly, transparent clinical trials can build public trust in vaccines that have been developed at record speed and under emergency conditions.
National governments, the EU, and COVAX (CEPI)f5be509ecaec should ensure public access to information about the funding they provide for Covid-19 vaccine R&D. This would ideally be done through easily accessible national public platforms, where all information would be gathered as open data. Data should include amounts of funding, types of funding mechanisms, the identities of recipients and providers, and conditions of funding agreements. Considering that the largest public funders are members of the Organisation for Economic Co-operation and Development (OECD), it would be worthwhile to link such national-level data to an international central registry: for example, the recently created website of the Task Force on Covid-19 vaccines, therapeutics and diagnostics (the Covid-19 Task Force).7add4edb6d32
National governments, the EU, and COVAX should require the pharmaceutical industry, including their partner research institutes, to publish information on the funding of Covid-19 vaccine development, indicating the ratio of public to private funding and details around research, clinical trials, and so on, up to the point of market approval.5fac875ccc43 The development of the Moderna vaccine, which was largely funded by the US government, could serve as a useful reference here, as Moderna was pressured by the US government to publish these data to avoid sanctions under US patent law.
National governments, the EU, and CEPI should also review their current practices with APAs based on the Principles on Commercial Transparency in Public Contracts, developed by a working group under the Center for Global Development in 2019. At a minimum, the number of doses committed and the indicative delivery schedule should be publicly available, while all redactions of the APAs should be justified.
Regarding clinical trial transparency, there is a need to develop specific provisions to be applied to trials of vaccines. In the context of pandemics, these provisions must strike a balance between speed, quality assurance, and the generation of public trust.826902a887e1 The following should be considered, irrespective of existing legal requirements for ‘normal’ (non-pandemic) situations:
- Vaccine developers should publish clinical trial protocols, at latest, at the start of the respective clinical trial.
- Vaccine developers should make clinical trial results public, at latest, when applying for market approval.
- National and regional drug regulatory agencies should make their complete clinical study reports available immediately upon approval of the vaccine, while ensuring data privacy of participants.
- COVAX should request transparency of clinical trials and help make the information publicly available.
2.2 Manufacturing
The current Covid-19 vaccine market can be described as a seller’s market, where an oligopolistic vaccine supplier structure with limited production capacity is facing an enormous and pressing demand for vaccines. As a result, the global demand for vaccines far outstrips the available manufacturing capacity of vaccine developers. Despite early alerts about a vaccine supply shortage, international initiatives to counteract this risk have not yet gained sufficient traction.
The spread of new virus mutations across borders and continents reinforces the need for a global approach to rapidly scale up Covid-19 vaccine production. Transparency and access to information are crucial ingredients for decision making in this regard. To enlarge manufacturing capacity, efforts must be made to ensure the required expertise, an adequate international supply chain flow, and quality control of the manufacturing process. But one of the most important issues is the need for transparent political choices: who can manufacture vaccines, where, using which technologies, and under what terms.
2.2.1 The current situation in manufacturing
One of the first international initiatives in the manufacturing space emerged in May 2020. The Costa Rican government, together with the World Health Organization (WHO) and with the support of 40 WHO member states, launched a proposal to openly share knowledge, intellectual property, and data on Covid-19 vaccines. Known as the COVID-19 Technology Access Pool (C-TAP), it is now an international platform housed at WHO.f75ed822775e The C-TAP mechanism has been presented as complementary to COVAX, but it has not yet received much attention or support from governments that are home to vaccine-developing pharmaceutical firms, from the firms themselves, or from Gavi.871b08039b26 The WHO Director-General, however, has said that the C-TAP can ‘overcome the artificial vaccine scarcity’ and that ‘waiving patents temporarily won’t mean innovators miss out.’021115b60cc4
It is not in the commercial interest of vaccine producers to open their patents and share their technological know-how through platforms such as C-TAP. In view of the international crisis, however, governments and COVAX should have pushed vaccine developers to do so, especially for those Covid-19 vaccines whose development has been mostly funded by taxpayers’ money, such as Moderna and Oxford/AstraZeneca.b55d7af4e301 Instead of using C-TAP, WHO donors are now working to create new technology transfer hubs under the Access to Covid-19 Tools Accelerator (ACT-A) – sidelining C-TAP without providing any justification. The lack of transparency around these hubs is problematic, although their creation is welcomed in general.a32d5425d417
In October 2020, India and South Africa proposed to the World Trade Organization (WTO) a waiver from some provisions of the Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS) for the prevention, containment, and treatment of Covid-19. There are, in fact, mechanisms in the TRIPS agreement itself that allow for this kind of property-rights suspension during public health crises. However, the proposal, which has been supported by close to 100 countries, has been consistently blocked by Australia, Brazil, Canada, the EU, Norway, Switzerland, the UK, and the US. These are the same countries which have secured the majority of currently available vaccines. In May 2021, President Biden changed the US policy position, announcing support for patent waivers. Since then, other countries and the Bill & Melinda Gates Foundation have also indicated a reversal of their initial resistance.
Vaccine developers, by contrast, have insisted that the problem lies in countries’ limited expertise and manufacturing capacity, which would pose challenges for technology transfer, they say. However, there are companies that do have vaccine production capacity. These include firms that have been unsuccessful in developing a vaccine themselves but could help manufacture them, as well as firms that are producing other vaccines and could produce millions of Covid-19 doses at short notice if they had the recipe. In some countries, including low- and middle-income countries (LMICs), Covid-19 vaccine production capacity could be set up within three to four months.3f79a932e6af
There have also been several initiatives from civil society calling for patent waivers and technology transfer as means of promoting equal and fair access to vaccines. One example is the People’s Vaccine Initiative, which is supported by political and economic leaders, health experts, and organisations around the world.
Finally, many Covid-19 vaccine developers who have entered into production agreements, such as AstraZeneca with the Serum Institute in India, have not published the conditions of the associated licensing agreements. Prices, delivery commitments, penalties for non-compliance, and other terms need to be publicly available. India’s vaccine manufacturers, for example, are producing Covid-19 vaccines for the world market under licensing agreements, and in particular for COVAX. However, in response to the sudden Covid-19 surge in India in March–April 2021, the country started prioritising the provision of vaccines to its own population. This has come at the expense of ‘Indian manufacturers’ ability to service contracts to supply vaccines for COVAX … and more clarity [is needed] on the implications of India’s export restrictions that are affecting supplies globally’.cfc3f786b99c Also, the lack of transparency is further eroding public trust in COVAX’s capacity to deliver.
There have been some isolated efforts to make licensing agreements public. For instance, the Brazilian public research body Fiocruz published its licensing agreement with AstraZeneca for the production of 100 million doses. This could serve as a reference for the future.
2.2.2 Manufacturing: What should be done?
Governments that have provided massive public funding for Covid-19 R&D need to demand that pharma companies accede to technology transfer as a means of scaling up worldwide vaccine supply.
Public funders of vaccine developers should publicly state their positions on issues such as temporary patent waivers and the different options for technology transfer to vaccine producers, mainly in developing countries. In particular, they should explain their reasons for supporting or not supporting C-TAP, especially for those vaccines that received public funds. They should require pharmaceutical companies – particularly those, like Moderna, that have publicly declared that they would not legally pursue others who copy their patents – to explain why they do not support or use C-TAP, and incentivise them to ensure that vaccine production by other companies, particularly in low-income countries, can actually happen.
National governments, the EU, and COVAX should require the pharmaceutical industry to make all Covid-19 vaccine licensing agreements public. In 2021, the WTO Secretariat took the first step in this direction.bfdea2366d35 While there may be legitimate commercial reasons to withhold certain licensing terms,1d4df733e322 all key stakeholders should reveal the information needed to ensure fair and equitable access to Covid-19 vaccines globally. This includes, among others, commitments on quantity and delivery schedules disaggregated by country and/or COVAX, price guarantees,b69e809d1cc4 and conditions for revising licensing agreements (for example, due to national Covid-19 outbreaks like the one in India). This information should ideally be hosted on the same national or international platform(s) mentioned in section 2.1.2.
High-income countries should actively support the scaling up of vaccine production globally. This should include a review of their current approaches in order to address the following apparent policy incoherence: First, large amounts of taxpayer money have been used to develop vaccines. Second, a significant amount of money is spent through overseas development assistance (ODA) to help the 92 poorest countries access Covid-19 vaccines through COVAX. However, the procurement contracts that COVAX has entered into are not transparent, nor is there any transparency around pricing. And third, public funds also go towards ODA to help LMICs address the economic, social, and broader development effects of the Covid-19 crisis in order to reach the Sustainable Development Goals (SDGs).
Despite this huge expenditure, many high-income countries have not supported proposals to push and/or incentivise technology transfer. Such transfer, leading to more widespread production of vaccines, could significantly reduce the need for public money to address the second and third points above. This argument seems to have been overshadowed by the emphasis on protecting intellectual property rights and the commercial interests of national pharmaceutical industries.
Finally, public funders of vaccine developers should ensure public access to their lobby engagements, and arrangements for dealing with conflicts of interests and beneficial ownership should be clear. For instance, existing lobby and beneficial ownership registers could include a specific sub-section for Covid-19 vaccine-related activities. The views and positions of the pharmaceutical industry regarding public policy decisions should be published in an accessible way, and information on members of Covid-19-related health technology committees, their professional backgrounds, and their declarations of conflicts of interests and beneficial ownership should be public and easily available.
2.3 International distribution
The international distribution of Covid-19 vaccines has been one of the most controversial issues in global efforts to address the pandemic. While the wealthiest nations have secured sufficient vaccines to inoculate their populations several times over, LMICs have had little access to Covid-19 vaccines.
2.3.1 The current situation in international distribution
By late May 2021, Canada, the UK, the EU, the US, and Japan had collectively procured 5.3 billion vaccine doses for a combined population of just over 1 billion, or 5.3 doses per person. In contrast, Colombia and Indonesia had enough to provide just 0.9 doses per person, South Africa 0.5, and Pakistan as little as 0.1 dose per person.4d1405e0fbea
Unequal access to vaccines is reflected in unequal vaccination rates. By September 2021, 45.2% of the world population had received at least one dose of a COVID-19 vaccine, and 33.9% were fully vaccinated. However, only 2.3% of people in low-income countries had received at least one dose.0f33e820e6af The US had a vaccination rate of 64%, the UK 71%, and Canada 77%. Meanwhile, the rates for Guatemala, Indonesia, Bangladesh, and Uganda were 24%, 32%, 19%, and 3%, respectively.07e840ad9b9c
Despite last year’s global pledge to ensure equitable and fair access to Covid-19 vaccines for all, vaccine nationalism and hoarding by high-income countries continues, and a number of middle-income countries have followed their lead. Instead of a global and collective approach to distributing vaccines, as initially foreseen through the COVAX facility, the real-world scenario has turned out to be a fierce competition among mostly high-income and some middle-income countries to lock down the supply.1bec4c61985e
In the European context, there are understandable tensions between national governments and the EU with respect to vaccine acquisition. EU countries are accountable first to their own populations, which expect full vaccination as soon as possible, especially if public funds were used for the vaccines’ development. At the same time, most governments have signed on to the pledge for global equal access to vaccines. The contradictory political incentives at play have led to a typical collective action problem: short-sighted, short-term, country-level solutions are prioritised over a more equitable distribution of vaccines across the world that would save more lives in the end.0f458d16c5ac
COVAX has a self-financing channel for middle- and high-income countries and a channel of subsidised vaccines for the 92 lowest-income countries. This is an unprecedented initiative to ensure equitable distribution, but its implementation has lagged behind the rhetoric. COVAX had difficulties from the start in securing vaccine purchasing agreements. Poor clinical trial transparency and slow submission of trial results by pharma companies and researchers, leading to delays in the granting of emergency use listing by WHO, have compounded the difficulties.30181277ca40
Likewise, bilateral deals between rich countries and the pharmaceutical industry have undermined the functioning of COVAX. Ironically, some of the governments accused of vaccine hoarding are also among the main funders of COVAX, including the US, the UK, and Canada. Many have recently pledged to donate large numbers of vaccine doses to ease vaccine inequity. But the question remains: Why have there not been more efforts to make COVAX work well from the start, for both global public health and economic reasons?cc4975918a64
Some critics have suggested that COVAX is basically an aid project for low- and middle-income countries rather than a true global collaboration to control the pandemic.b2c75478bd46 The recent commitments of G7 countries to donate 1 billion vaccine doses could be read in that light, as their donations seem to draw mainly on their own oversupply. While generally welcome, the pledges have been criticised4df36bec9dfd for falling short of the 5 to 6 billion doses needed by poorer countries. It remains unclear why not all donated doses are channelled through COVAX to ensure fair universal access,e850ff4bc46e and what criteria governments are using to decide how many doses they allocate to which countries. For these reasons, it is doubtful that this ‘vaccine diplomacy’ is in line with the global commitment to fair and equitable distribution.
COVAX’s vaccine supply shortages have seriously affected many of the poorest countries, especially on the African continent. But COVAX has not openly communicated the challenges it faces in living up to its promises. A transparent and open review of the limitations of the current COVAX mechanism is crucial, although COVAX appears unwilling to institute one.a10e2b80486d In a tightly interconnected world, with emerging virus mutations that spread more easily than earlier forms, the pandemic is highly unlikely to be contained with the current vaccine distribution situation. A transparent debate on this dilemma is overdue.
The Covid-19 Task Force is a step in the right direction. Established in June 2021 as a collaboration between the International Monetary Fund, World Bank, WHO, and WTO, the Task Force will track, coordinate, and advance delivery of COVID-19 vaccines, therapeutics, and diagnostics, working with governments and partners to address finance and trade barriers. It aims to vaccinate 40% of the world population by the end of 2021 and 60% by mid-2022. July 2021 saw another positive development, with the World Bank and COVAX announcing a new financing mechanism to increase the Covid-19 vaccine supply for developing countries. And suggestions for the creation of fair and transparent distribution principles to be taken into consideration by companies are being developed. However, it is unclear how these commitments will be achieved, especially since high-income countries are considering booster doses for their populations, and some, including the United States and Germany, have started distributing them.
In addition to the international economic, moral, and human rights dilemmas, there is a more pragmatic issue for the wealthy countries. What impact will their political positions regarding Covid-19 vaccines have on their standing in development cooperation with their partner countries? How will the performance of OECD countries compare to that of new or non-traditional donors like China, India, and Russia? Countries such as China and Russia are also engaging in Covid-19 vaccine diplomacy. Moreover, these countries also provide development funding with few or no conditionalities regarding good governance or human rights. The implications of these shifts remain to be seen.
2.3.3 International distribution: What should be done?
It is crucial that information be made public at the global level about countries’ access to vaccines, the number of doses promised and/or delivered, the delivery schedules, and the prices paid. This information is needed whether vaccines are delivered through bilateral agreements, COVAX self-financing, subsidised channels, or bilateral donations. Towards this end, a global clearinghouse of publicly accessible information on vaccine supply and demand is needed. It would provide a basis for concerted global efforts to balance out inequities in vaccine access and inform other decisions for a more effective global response to the pandemic. Political support, especially from the vaccine-producing countries and those able to donate vaccines, would be instrumental in ensuring its effectiveness.
Such a clearinghouse should include information on the current situation along with projections for the next six months. This should be done in open data format and include data on vaccines contracted through all four channels mentioned above. The Covid-19 Task Force, which launched a website on vaccines, therapeutics, and diagnostics in July 2021, provides some of the suggested data. Links between the Task Force and COVAX should be explored. There are, as well, other public databases with partial information that could feed data into a central clearinghouse.be0d547f964b Thus it is important to explore whether the website of the Covid-19 Task Force could be expanded to accommodate the proposals described above and to allow others to join their efforts.
Recognising that the development of a clearinghouse will take time, the following actions should be taken with immediate effect and in such a way that they could later feed into the global clearinghouse on vaccine demand and supply:
- All governments, the EU, and other vaccine buyers should publish the following information in open data format: (a) number of doses secured and delivery schedules through bilateral deals (APA or fully contracted); (b) number of doses received through bilateral deals; (c) number of doses demanded through COVAX (self-financing channel); and (d) number of doses received through COVAX (clearly indicating if through self-financing or subsidised channels). Points (a) and (b) shall be verifiable using information published by COVAX (see below). This information would ideally be published by countries on their own central Covid-19 vaccine webpages (see sections 2.1 and 2.4).
- COVAX should publish the number of vaccine doses it secures from each supplier, through both APAs and final contracts, indicating the delivery schedules to which vaccine producers committed.
- COVAX should also publish, in open data format and in real time, information on its vaccine distribution activities, including the number of doses committed and the delivery schedules for each country, as well as the actual in-country delivery of vaccines, differentiating between self-financing and subsidised channels (something which does not seem to happen currently). This information shall be verifiable using the information published by individual countries mentioned above.
- Governments and the EU should also publish, in open data format and in real time, information about their donations of vaccines, how the donations will be handled (either directly through bilateral agreements or through COVAX), the criteria used to allocate donations to recipient countries, as well as the sources of the donations (for instance, donation of surplus doses from current stockpiles versus contracting of new production).
Finally, the proposal for an international treaty on pandemics, which aims to build on lessons learned from the Covid-19 crisis, seeks to ensure greater transparency, accountability, and shared responsibility in the international system. This includes the issue of international access to and distribution of vaccines.
2.4 Contracting
Transparency in Covid-19 vaccine contracts is key for strategising, planning, and budgeting national vaccine roll-outs. It is also a crucial prerequisite for public accountability, public scrutiny, public trust, and the prevention of corruption and conflicts of interests. Major challenges include publication of contracts and the level of redaction; information on prices; quantity of doses procured; delivery schedules; and liability and indemnification.
2.4.1 Publication and redaction of contracts
Transparency in the publication of public contracts, including information about prices, quantities of goods, delivery schedules, risks, and liabilities, is the basis for evidence-based decision making in procurement. Transparency also enhances public scrutiny, which in turn helps to minimise corruption. Many countries have subscribed to the Open Contracting Partnership; some participate in or fund the Open Contracting for Health Initiative; and most countries have freedom of information laws in some form. These initiatives mandate transparency in public contracts, which naturally extends to health technologies and Covid-19 vaccine contracting. Calls for Covid-19 contract transparency are further based on the premise that citizens have a right to know the details of such contracts,0b332af1427c especially since the vaccines have been developed with large amounts of public funding. Finally, the Inter-American Commission on Human Rights (IACHR) urged member states in its recent resolution to ensure transparency and access to information in relation to Covid-19 vaccine procurement and distribution.
As of March 2021, most Covid-19 vaccine contracts were not publicly accessible, according to a report by Transparency International Global Health (TI-Global Health). The report examined 183 agreements between 75 governments and 13 suppliers for the purchase of 12 different vaccines. Of the 183 agreements, only 11 were officially published, while two others were leaked. The 11 officially published agreements were made available by the US, the EU, the UK, Brazil, and the Dominican Republic. As an exception, the US formally published all six contracts it had signed.776343502dbe
Freedom of information (FOI) requests relating to vaccine contracts, generally submitted by civil society organisations (CSOs) or the media, have been denied in many countries. CSOs have used legal strategies to insist on the fundamental right of access to public information, including through national justice systems. In many cases these have resulted in legal counteraction by governments appealing to higher-level judicial instances for a decision on the FOI requests. In a few cases, contracts have been released by accident, as happened recently in Colombia.
Those contracts that have been proactively published by a handful of governments are heavily redacted, as are the EU’s advance purchase agreements. The redactions, according to TI-Global Health, often cover ‘entire pages and sections, as well as information of key public interest, such as the total cost paid, the price per dose, and delivery timetables.’ In some cases even crucial definitions of contract obligations, such as what is meant by the term ‘best possible effort’ (to ensure delivery according to the agreed schedule), are redacted. The report found only one contract – the one between the Dominican Republic and Pfizer, which was released in response to an FOI request – to be entirely free of redactions. The United States was the only country that justified its redactions based on a legal provision.
Building on the work initiated by Transparencia Mexicana (TM), the Mexican Chapter of TI, and TI-Global Health, TM has recently examined 37 Covid-19 vaccine contracts from 14 countries and the EU that were publicly available as of September 2021.8d249d8d4e39 The results of this in-depth analysis confirm, by and large, the mentioned secrecy of information and show no significant difference between rich and middle-income countries. Exceptions to this pattern are the contracts that have been leaked, like in the case of Colombia.
Figure 2. Low level of transparency in 37 Covid-19 contracts from 14 countries and the EU
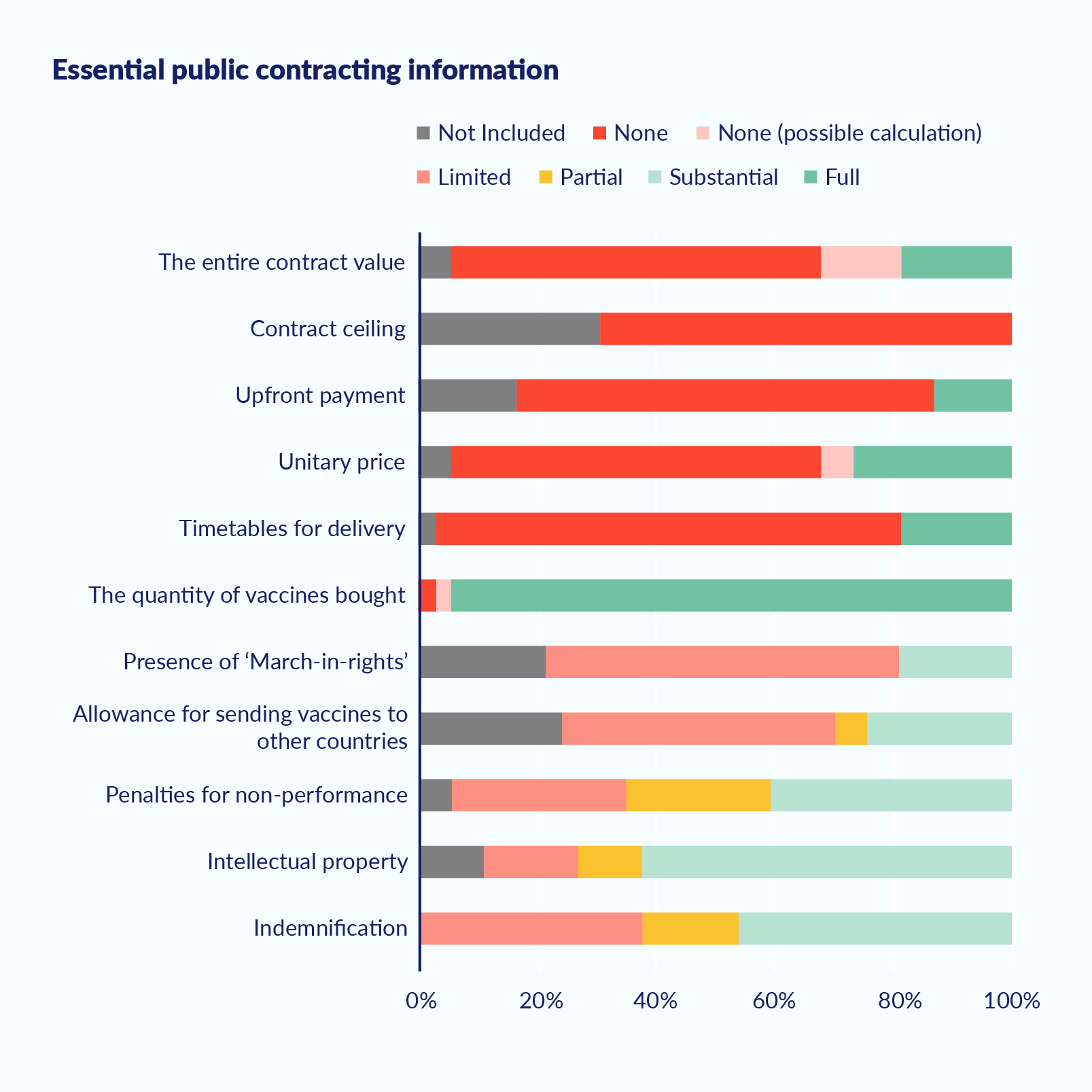
Source: Transparencia Mexicana (publication upcoming).
The secretive practices around Covid-19 vaccine contracting are out of line with the Principles on Commercial Transparency in Public Contracts, and with past practice. Redaction has been much less common in contracts for other types of vaccines.f93a8502a012 Many governments justify redacting material, or denying access to Covid-19 contracts entirely, by pointing to the need to protect commercial or industrial secrets. They also note the risks of breaching confidentiality clauses they have agreed to under pressure from pharmaceutical companies, which they fear might cause them to be excluded from future vaccine contracts. There is growing civil society pressure to defy these arguments, however, as protecting the public interest should be more important than protecting private interests in the current health emergency.
2.4.2 Prices, doses, and delivery schedules for Covid-19 vaccines
Transparency in vaccine prices is key to ensuring that LMICs have equitable and sustainable access to life-saving vaccines. Price transparency helps inform public procurement processes, enabling officials to improve budget analysis and analyse purchasing choices.7e75287c074b Globally, public information on vaccine prices can reveal potential market distortions and inequitable access. This information can be used to compare prices paid by governments with national gross domestic product (GDP) and/or national public budgets.d6e33d33bc59 Finally, the 2019 WHO resolution on improving the transparency of markets for medicines, vaccines, and other health products urges governments ‘to take appropriate measures to publicly share information on the net prices of health products.’
Secrecy around vaccine pricesbee1ec78d692 has contributed to some LMICs paying more for Covid-19 vaccines than high-income countries. For instance, according to the Center for Global Development, ‘the European Commission is paying $2.19 per dose for AstraZeneca’s vaccine compared to $5.00 for the Philippines or $5.25 for South Africa. The African Union is paying $10 for the Janssen vaccine compared to Europe’s $8.50.’e64728e300a2 Price variations for the AstraZeneca vaccine are illustrated in Figure 3, based on data from Unicef’s Covid-19 Vaccine Market Dashboard.28a5b94ef048
Figure 3. Price paid per country for full course of AstraZeneca vaccine (US$)
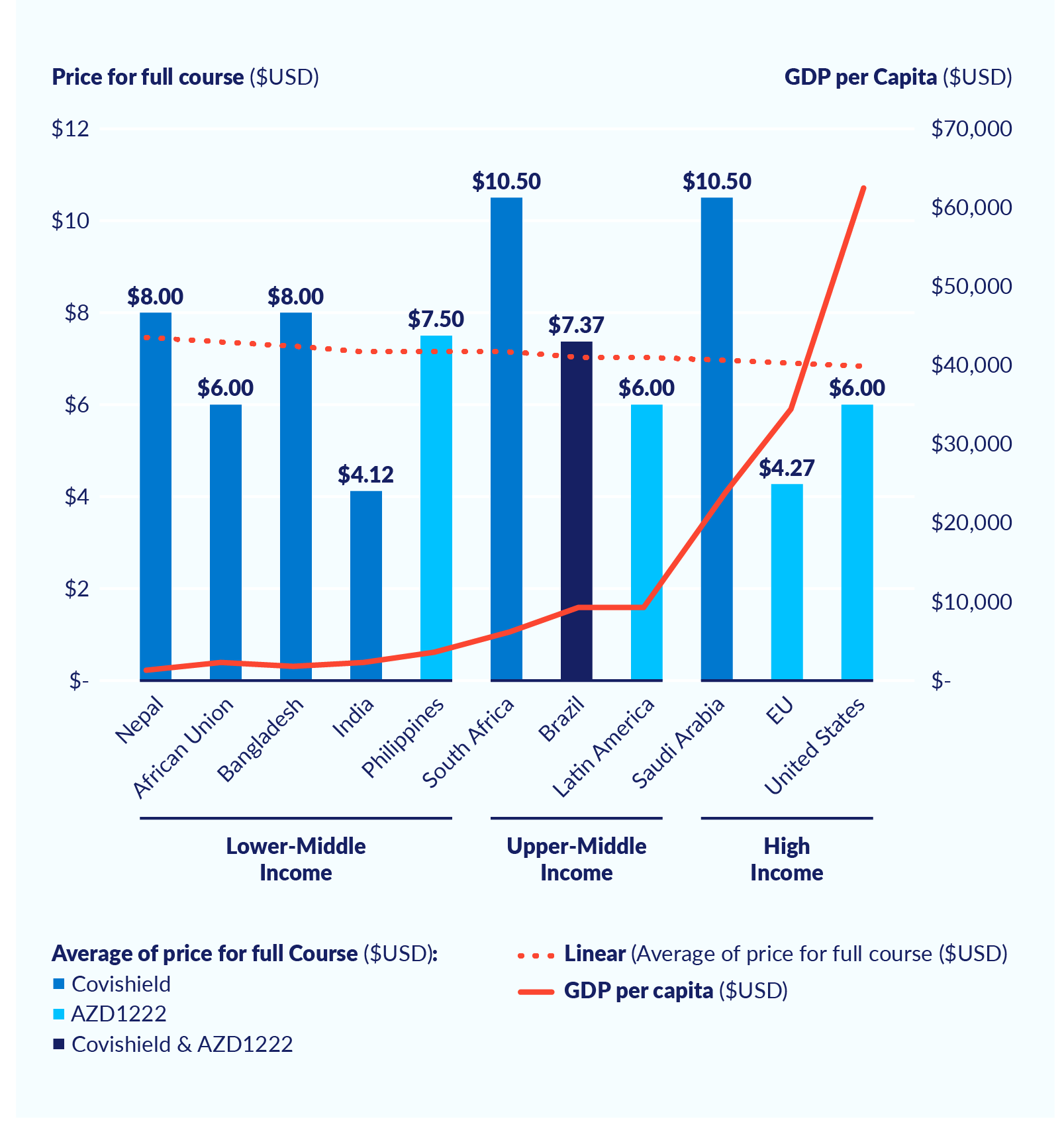
Source: Transparency International Global Health, May 2021.
Figure 4 shows the significant differences in the prices that countries pay for a full course of Covid-19 vaccines, taking all available vaccines into consideration. Why are LMICs on average paying higher prices than wealthier countries? Why is it that Senegal and Ukraine purchased some of the most expensive vaccines? Do prices reflect the negotiating power of countries, relative to the pharmaceutical companies? What needs to be done to level the playing field to allow for fair and equitable access to vaccines for all countries?
Figure 4. Price paid per country for one full course of Covid-19 vaccine (US$; all vaccines)
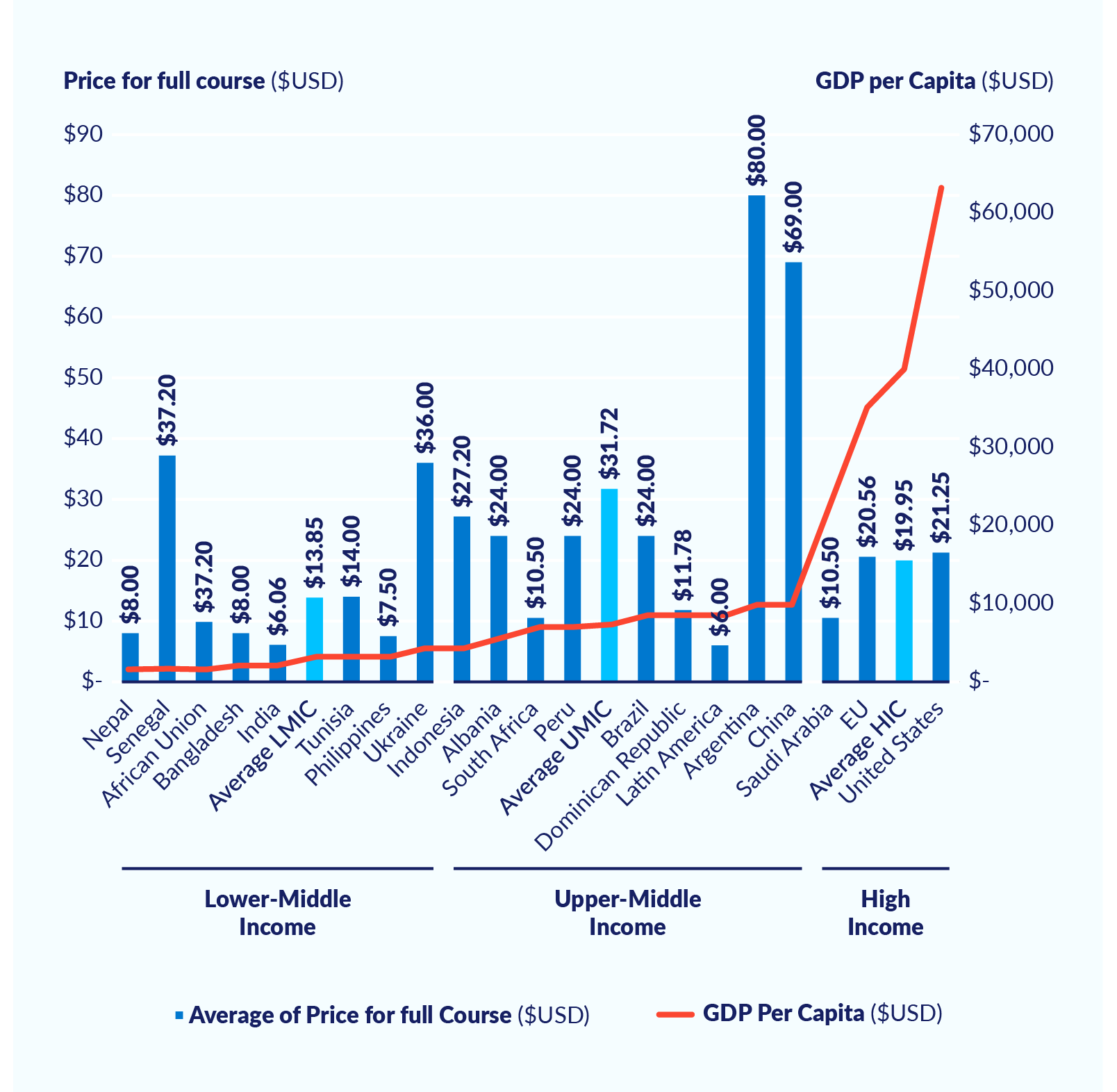
Of course, prices may vary across countries for legitimate reasons related to volume, differential costs of obtaining regulatory and marketing approval, expenses related to country-specific labelling requirements, different commercial and liability risks, and political risks. However, transparency is needed so that the public can be sure that pricing discrepancies are indeed legitimate.
Public information on the number of vaccine doses purchased and on delivery schedules is crucial for the strategic planning and actual implementation of national vaccination campaigns. To build public trust and legitimacy, governments need to show that they are delivering on their promises. This data is also indispensable to ensure fair and equal global access.
Information about number of doses delivered seems generally more available than information about delivery timetables. The latter is mostly redacted in the published contracts, as well as in the APAs. In addition, there is little to no information about penalties for non-performance, that is, for failure of the developer or manufacturer to deliver on their obligations. There are real uncertainties in the complex Covid-19 vaccine production process, with its multifaceted globalised supply chains. Shortages of inputs and other such problems can constitute legitimate reasons to adjust the delivery schedules. Yet the extreme secrecy surrounding this issue is unacceptable, given its negative impact both on national vaccination programs and on global fair access.
Another issue is whether contracts allow the procuring country to either donate or resell unused doses, and under what conditions. This is of specific relevance for those countries that have stockpiled far too many doses. G7 countries announced in June 2021 that they will donate close to a billion vaccine doses from their oversupply. How this is implemented going forward can provide insights into the question of conditions for donation and resale.c647c2d0c9d4
2.4.3 Indemnity clauses
Liability and indemnity clauses seek to reduce the risks producers may incur because of unknown, potentially adverse effects of the new vaccines. These clauses are especially relevant during pandemics, when the long processes of normal vaccine development need to be considerably shortened. However, the public needs to have full knowledge of what their governments are agreeing to when they take on these risks from the producers.
While there are legitimate reasons for vaccine contracts to include indemnity clauses, the clauses need to be made public and open to scrutiny. However, only three of the 13 published or leaked vaccine contracts analysed by TI-Global Health contained unredacted information on indemnity. According to the report, there are indications that indemnity provisions in vaccine contracts of high-income countries are more limited in scope than those of LMICs. This may be the result of LMICs’ weaker negotiation power and legal capacity. For example, the Pfizer contract with the Dominican Republic goes beyond the ‘normal’ protections against legal and financial liabilities to include issues that may arise with dispensing and administration of the vaccines.06c3377c6891 Such indemnities mean that LMICs have to cover broader risks with lower financial capacity to do so. Access to information on these clauses is needed to ensure that future contracts, concluded by either governments or COVAX, are negotiated in a standardised and fair way for all.
2.4.4 The COVAX facility
COVAX-funded investments and vaccine contracts have also been conducted in secrecy. In a recent blog, Human Rights Watch, Amnesty International, and Public Citizen stated that COVAX ‘has not yet published details about procurement pricing and profits in its agreements with vaccine developers and manufacturers.’ In response to pressure from civil society, COVAX argued along the same lines as many governments, saying that the contracts ‘contain commercially sensitive and proprietary information protected under confidentiality obligations.’
Considering that COVAX is a multilateral collaborative effort to ensure fair and equitable distribution of vaccines, this situation is alarming. It is striking that COVAX is not yet fully aligned with the long-standing practice of UNICEF on price transparency, given that UNICEF is one of the main COVAX partners for Covid-19 vaccine distribution.446416546185
Pharmaceutical companies are undoubtedly exerting pressures on COVAX to accept their confidentiality conditions. It is unclear, though, why participating governments and donor agencies, in particular those providing the largest amounts of funds, do not flex their political and financial muscle to make the disclosure of contract information mandatory.
2.4.5 Contract transparency: What should be done?
All governments and COVAX should make their Covid-19 vaccine contracts publicly available on their respective public procurement platforms. Ideally, key data points should be published in structured open data formats, with the information linked to a central international database or clearinghouse for easy access (see section 2.3.3).
COVAX member states should forcefully demand that COVAX contracts be made public. Those countries funding COVAX subsidies for LMICs should throw their political weight behind this approach and facilitate dialogue with pharmaceutical companies to this effect.
Governments, the EU, and COVAX should ensure that redactions of contracts, as well as of APAs, are kept to a minimum. Where redactions are made, they need to be justified. The Principles on Commercial Transparency in Public Contracts should be applied.
In line with the 2019 WHO resolution on pricing transparency, governments, the EU, and COVAX should publish the price paid per dose and clarify whether that price included the cost of any other part of the delivery chain. In the case of COVAX, special efforts should be made to ‘verify all commitments by companies to supply COVAX at non-profit prices or minimal profit pricing through third-party audits whose results are publicly shared.’b935711b79c0 Such information should be gathered in a central and easily accessible database and should eventually be part of the suggested Covid-19 vaccine clearinghouse of information (see section 2.3.3). Liberal democracies, including EU countries, the UK, the US, and others, should champion price transparency to facilitate price comparisons worldwide.
Governments, the EU, COVAX, and other vaccine buyers should publish the quantity of doses procured as well as the delivery schedule for each contract (see section 2.3.3), along with the conditions and the potential sanctions for supplier non-performance (see section 2.4.2).
Governments, the EU, and COVAX should also publish full information on liability conditions and indemnity clauses, ideally in agreement with the vaccine suppliers. Countries with strong negotiating positions, typically the high-income countries, should take the lead by providing this kind of information so that it can be used as a reference point by countries with reduced bargaining power. As suggested by TI-Global Health, the available information on indemnity and liability clauses should be used to develop standardised ‘template clauses and guidance on good practices’, similar to the work that was done by WHO during the H1N1 pandemic.
3. Recommendations for donor agencies and international organisations
The Covid-19 pandemic has been characterised by record-speed vaccine development but grossly inequitable global distribution of vaccines. The international community must do more to ensure universal and equitable access to these life-saving drugs. They should do so first and foremost out of self-interest: the virus and its dangerous mutations currently threaten new waves of infection even in countries with the highest vaccination rates, as happened in mid-2021 in Israel. The international community also has an obligation to ensure the human right to health, as well as other economic and social human rights. High-income countries must tackle the inconsistencies and contradictions in their own policies and actions and guarantee that protection of their national pharmaceutical industries does not undermine their international commitments to advance the Sustainable Development Goals.
While many of the issues discussed in this paper have been raised in different forums, efforts to actively enhance transparency have been disjointed, focusing on partial aspects of the vaccine value chain. Changing course requires going beyond business as usual. Transparency is indispensable to fully understanding what has been done in this crisis, how, and why. Learning from the mistakes and successes in dealing with the current pandemic will help inform responses to similar public health crises in the future.
Towards this end, donor agencies and international organisations, including the World Health Organization, the World Bank, the International Monetary Fund, UN agencies, and others, should consider the following recommendations.
3.1 Ensure policy coherence for sustainable development in line with SDG 17
Donor agencies should aim for policy coherence in relation to their home countries’ international Covid-19 vaccine response, in line with SDG 17 (‘Partnerships for the Goals’). This involves working to ensure that their home country trade, commercial, and pharmaceutical contracting policies related to Covid-19 vaccines do not contradict or undermine their international development policies and commitments. Donor agencies can ask their own governments to do the following:
- Actively disclose key information in an easily accessible, open data format, covering:
- Amounts of taxpayer money invested in Covid-19 vaccine development, type of funding mechanism (e.g., loan, grant, credit, APA), type of recipient (e.g., biotech firm, university, pharma company), and conditions of the funding agreement (e.g., future tech transfer), among others;
- Access to vaccines (number of doses secured, projected delivery, distribution plans for excess doses);
- Vaccine contracting information, including on APAs (see below).
- Promote C-TAP, the COVID-19 Technology Access Pool, as a platform for sharing data and technology on Covid-19 vaccines with a view to scaling up vaccine production, especially in developing countries. Efforts could start with the Moderna vaccine, as the company has stated it will not initiate legal action against any manufacturer using its ‘recipe’.00252a207bc2 International organisations should join such efforts.
- Foster public debate about incoherent policy approaches. High-income countries are spending large amounts of taxpayer money on vaccine development; then more taxpayer money to purchase vaccines through COVAX, whose procurement contracts and vaccine prices are not transparent; and still more taxpayer money on overseas development assistance to help LMICs cope with the Covid-19 health crisis. They do all this while failing to incentivise vaccine developers to allow technology transfer, which could significantly reduce public expenditure on both vaccines and ODA.
- Guarantee openness and public access to information about lobbying activities, beneficial ownership, and conflicts of interests between the pharmaceutical industry and other political and economic actors with stakes in the Covid-19 vaccine process.
- Actively promote public contracting in line with corresponding international commitments. This should include:
- Publication of vaccine contracts (including APAs);
- An absolute minimum of redactions, which would need to be legally justified;
- Price transparency;
- Open information about the number of doses and delivery schedules;
- Fully published indemnity and liability clauses.
- Provide open and transparent vaccine donation plans, including publication of the criteria used for allocation, particularly if the donations are not channelled through COVAX. This would promote fair and equitable access to vaccines rather than ‘vaccine diplomacy’.
3.2 Promote transparency in all COVAX’s crucial functions and processes
- Demand from COVAX (CEPI) proactive disclosure of information, in an accessible manner and open data format: for example, information on amounts of funding given to which vaccine developer, through which type of funding mechanism, and under what conditions.
- Demand from COVAX proactive disclosure of vaccine contracts (including APAs) with an absolute minimum of redactions, which would need to be legally justified; price transparency and audits to verify not-for-profit prices; open information about the number of doses secured and delivery schedules; as well as fully published indemnity and liability clauses.
- Demand that COVAX make public the criteria used to allocate vaccine donations it has received from the excess stockpiles of wealthier countries, as well as delivery times.
3.3 Support the development of an international clearinghouse of information on Covid-19 vaccines
- Provide financial and political support for the creation of an international clearinghouse of information on all international dimensions of Covid-19 vaccines, including R&D, demand, supply (manufacturing), procurement, and distribution. Such an effort should build on existing initiatives and integrate the fragmented information and database landscape into a comprehensive ‘one-stop shop’ platform.
3.4 Support civil society organisations and academia in their research and engagement with the public
- Provide financial and political support to CSOs and academia to research the roles of public versus private funding for vaccine R&D, the dynamics of public-private partnerships, and the associated political economy to capture lessons for better pandemic planning and preparedness.
- Support CSOs to engage with the pharmaceutical sector on transparency initiatives, especially for vaccine development, manufacturing, and distribution. This should include building linkages to transparency, integrity, and human rights principles, such as the UN 2008 Human Rights Guidelines for Pharmaceutical Companies in Relation to Access to Medicines.
- Support CSOs and academia to systematically analyse public debates on controversial policy issues regarding Covid-19 vaccine development and the scaling up of production and international distribution. CSOs and academics can then disseminate this information to citizens, fostering public trust in the vaccines.
- Help amplify the voices of CSOs, ‘think and do tanks’, local industry associations, citizens, and others, especially those in developing countries, in international policy debates on Covid-19 vaccines.
3.5 Contribute to the generation and sharing of knowledge and lessons learned for future pandemics
- Critically analyse the international response to the Covid-19 crisis in general, and the vaccines in particular, in order to examine the policy options chosen, their underlying assumptions, and how they have played out in practice. The 2021 report of the G20 High Level Independent Panel on Financing the Global Commons for Pandemic Preparedness and Response provides a thorough analysis and recommendations on global pandemic preparedness. Improved transparency, open data, and accountability are identified as cross-cutting issues.
- Support the generation and sharing of knowledge and lessons learned. One key area for review is the accountability structure, principles, and practice of the international vaccine response ‘system’.e74f1d4985ee Equally important is an open and frank review of COVAX, with emphasis on the political economy dynamics that affect its role.
3.6 Support the development of minimum transparency standards for vaccines in pandemics
- Support the development of minimum transparency standards for vaccine R&D, manufacturing, and distribution, especially for vaccines needed to contain health emergencies. This should involve relevant actors from governments, multilateral agencies, the pharmaceutical industry, biotech research institutions, academia, and civil society. These standards should be established at both global and national levels. Throughout the Covid-19 response, rushed decision making and power asymmetries between different stakeholders have contributed to the neglect of transparency and access to information. The negative effects of this opacity and secrecy on vaccine equity should be avoided in future pandemic responses.
- The German minister for international cooperation referred to this situation of simultaneous crises as a ‘polypandemic’.
- On funding to vaccine developers, see CEPI criticised for lack of transparency. As an example of the wider pattern of contracting opacity, see Covid-19: Government has spent billions on contracts with little transparency, watchdog says, and the Transparency International Global Health report For whose benefit? Transparency in the development and procurement of COVID-19 vaccines, May 2021 (hereafter cited as ‘TI-Global Health report’).
- Open contracting for the Covid-19 vaccines: A good practice guide, Open Contracting Partnership and Transparency International Global Health, 2021.
- See, for example, The era of vaccine diplomacy is here and U.S. Covid-19 'vaccine diplomacy' is catching up to China and Russia's efforts.
- See, for instance, ‘V.I.P. immunization’ for the powerful and their cronies rattles South America; Latin America elites seek corrupt access to vaccines; and The strange preferential treatment of an Italian luxury hotel in Germany.
- Other crucial parts of the vaccine value chain, including the national roll-out of vaccination campaigns, have been covered in other publications, including a U4 Helpdesk Answer on mitigating corruption risks in Covid-19 vaccine roll-out and a UNODC paper on Covid-19 vaccines and corruption risks.
- COVID-19 vaccine R&D investments, last updated 8 July 2021. APAs are defined here as ‘agreements signed before vaccine approval by a Stringent Regulatory Authority … and for which there is data on the amount invested.’ APAs ‘were made before there was certainty in safety and efficacy of the vaccines and could be understood as an additional incentive that reduces business risk in the R&D stage.’
- The kENUP Foundation describes the role of APAs (also called advance market commitments or AMCs): ‘In return for the right to buy a specified number of vaccine doses in a given timeframe, governments finance part of the upfront costs faced by vaccines producers’ through APAs or AMCs.’
- See, for example, European Commission, EU vaccines strategy: Securing access to vaccines.
- COVAX, for instance, under the aegis of their funders and members, seem to have chosen to give incentives to participate in the mechanisms rather than looking for a multilateral binding accord on vaccine access and distribution. To what extent this may have been due to the global political economy of the time should be explored in future studies.
- In March 2021, CEPI published Summary of equitable access provisions in CEPI’s Covid-19 vaccine development agreements. Although this is a comprehensive account outlining CEPI’s engagement with different partners for vaccine development, scale-up of manufacturing, and supply of vaccine, key information is still missing, such as prices paid, contract liabilities, information on whether CEPI retains a public health license with partners tasked with vaccine development, and so on.
- See Carl Heneghan, BMJ Blog, 2 November 2018: ‘Clinical trials transparency means ensuring clinical trials are recorded in a publicly-accessible registry, summary results are published within a set time upon trial completion (usually 12 months) and results are reported in full.’
- See Boletin #23 of DIME on clinical trials and related issues.
- TI-Global Health report.
- The T-I Global Health report says in this regard that ‘press releases and press conferences enable companies to sequence information releases alongside stock movements, gaining a potential opportunity for private profit.’
- Such calls have been made in different forms by President Biden, the EU Parliament, civil society organisations like Human Rights Watch, Médecins Sans Frontières, and Transparency International, as well as former Nobel laureates and heads of state.
- Public scrutiny in this regard refers to specialised civil society organisations, media, and/or academic institutions that have the capacity and knowledge to inspect and analyse relevant information and package the results into a language easily understandable for ordinary citizens.
- Ann Danaiya Usher, CEPI criticized for lack of transparency, The Lancet, 2021.
- The Covid-19 Task Force website launched on 30 July 2021, one month after the Task Force itself was announced. Alternative repositories for a central registry should be explored and could include the OECD, with its data management capacity.
- See, for example, Human Rights Watch Report on Covid-19 vaccines, October 2020.
- See, for example, Elaine Ruth Fletcher, WHO calls for pharma transparency in clinical trial data reporting, Health Policy Watch, May 2021.
- C-TAP is implemented through the WHO Medicine Patent Pool, whose original mandate was to accelerate affordable access to quality treatments for HIV-AIDS and some other transmissible diseases for low- and middle-income countries.
- G7 countries have not made any pronouncements in favour of supporting C-TAP as a means of addressing the pandemic; see Amnesty International, press release, 19 February 2021, G7 leaders are shooting themselves in the foot by failing to tackle global vaccine access. Gavi does not even make reference to C-TAP in its article, Are vaccines a global public good? In February 2021, however, the leaders of the G7 did call for an international treaty on pandemics.
- See Nabil Ahmed, A shot of hope: Why a ‘people’s vaccine’ is central to our collective security and the economic recovery, Forum Network, March 2021.
- AstraZeneca, for example, stated in 2020: ‘Today’s announcement is not anticipated to impact the Company’s financial guidance for 2020 as expenses to progress the vaccine are anticipated to be offset by funding by governments and international organisations.’ The Moderna vaccine was largely funded by taxpayer money. Public Citizen said, ‘This is the people’s vaccine. The NIH [National Institutes of Health] vaccine … Federal scientists helped invent it and taxpayers are funding its development … It should belong to humanity.’
- Priti Patnaik, South Africa bags first mRNA tech transfer hub, Geneva Health Files, 22 June 2021.
- See, for example, The Economist Podcast ‘Can distribution be fair?’ and Vaccine makers say coronavirus could be stopped around the globe in months rather than years.
- Priti Patnaik, India: The quagmire for COVAX; Q&A: Hyo Yoon Kang on the financialization of intellectual property & COVID-19, Geneva Health Files, 20 April 2021.
- ‘In response to interest from members, the WTO Secretariat presented an initial compilation of data on the number of production arrangements under which vaccine developers had contracted vaccine manufacturing to other companies, and on the projected and actual production of COVID-19 vaccines under such agreements.’ World Trade Organization, Members approach text-based discussions for an urgent IP response to COVID-19, 9 June 2021.
- There continue to be debates at global and country levels as to the reasons that should be considered legitimate: for example, whether pricing strategies should be reserved. Accepted reasons are often restricted to national security concerns, usually related to the defence sector.
- Some vaccine producers, including those whose vaccine development was fully publicly funded, seem to reserve the right to define when the pandemic will be ‘over’ – that is, the point at which they would have the right to increase the vaccine price to non-pandemic prices.
- The Guardian, Share vaccines or climate deal will fail, rich countries are told, 5 June 2021. Informationbased on data collected by the Global Health Innovation Center at Duke University.
- Our World in Data, Coronavirus (COVID-19) Vaccinations; United Nations Development Programme (UNDP), Global Dashboard for Vaccine Equity.
- Our World in Data, Coronavirus (COVID-19) Vaccinations, accessed 29 September 2021.
- For a visualisation of existing data on vaccine equity, see the UNDP Global Dashboard for Vaccine Equity.
- A study at Northeastern University found that if rich countries monopolize Covid-19 vaccines, it could cause twice as many deaths as distributing them equally. ‘In one scenario, approximately 50 high-income countries monopolize the first 2 billion doses of the Covid-19 vaccine. In the other, doses are distributed everywhere based on each country’s population, not its ability to afford the vaccine. The models found that 61 percent of deaths could be averted if the vaccine was distributed to all countries proportional to population, while only 33 percent of deaths would be averted if high-income countries got the vaccines first.’
- Jenny Lei Revelo, Is COVAX part of the problem or the solution? Devex, 11 March 2021.
- As a study from 2020 indicated: ‘Even if nationalistic behaviour is inevitable, there are economic incentives to providing access to vaccines across the globe. Based on estimates by Oxfam International in 2020, it would cost $25 billion to supply lower-income countries with vaccines. The US, the UK, the EU and other high-income countries combined could lose about $119 billion a year if the poorest countries are denied a supply. If these high-income countries paid for the supply of vaccines, for every $1 spent, high-income countries would get back about $4.80.’ RAND Corporation, The global economic cost of COVID-19 vaccine nationalism, 2020.
- Jenny Lei Revelo, Is COVAX part of the problem or the solution?; Ann Danaiya Usher, Decolonise COVAX: An African critique, Development Today, 13 July 2021.
- Elizabeth Piper and Kate Holton, ‘We need more’: UN joins criticism of G7 vaccine pledge, Reuters, 12 June 2021; BBC News, Coronavirus G7: Could a billion more vaccines for poorer countries make a difference?, 14 June 2021.
- For example, in the case of the UK, allegedly 20% of the 100 million doses will be donated bilaterally (see ‘We need more’: UN joins criticism of G7 vaccine pledge). In the case of the US, 25% of the initially promised 80 million doses are channelled bilaterally. See Noah Weiland, Inside the Biden administration’s scramble to share vaccine with the world, New York Times, 30 June 2021.
- Priti Patnaik, #WHA74 WRAP: Pandemic Treaty Talks Eclipse Prevailing Vaccine Inequities, Geneva Health Files, May 2021.
- For example, OurWorldinData, the Unicef Covid-19 Vaccine Market Dashboard, and the UNDP Global Dashboard for Vaccine Equity.
- Since 2020 several specialised civil society and human rights organisations have called for transparency in this regard, such as Human Rights Watch, Médecins Sans Frontières, Oxfam, and Transparency International.
- T-I Global Health report.
- The publication of this research by Transparencia Mexicana is upcoming.
- The T-I Global Health report found that the UK published three comparable non-Covid-19 contracts for vaccines in 2019. Comparing the numbers and pages of redactions gave a striking result: there were between 2 and 8 redactions in each of the pre-pandemic contracts (on 2 to 4 pages) against 98 redactions in the UK–AstraZeneca deal (across 22 pages).
- The T-I Global Health report provides the following example: ‘In 2012, health authorities of Latvia, Estonia, and Lithuania used pricing data sourced from the Market Information for Access to Vaccines (MI4A) database to inform their joint tender for the rotavirus vaccine. This resulted in a lower price of 17–25 per cent for each immunisation course.’
- It is crucial to provide sufficient information so that comparisons are valid. For example, Management Sciences for Health has a website which reports drug prices paid, listing the volume, packaging, and delivery conditions, so that legitimate reasons for price differences become visible.
- ‘No company has shared information on research and development, clinical trials or manufacturing costs of potential COVID-19 vaccines, [Médecins Sans Frontières] said, adding this was vital for the public to assess prices set.’ Stephanie Nebehay, Activists urge ‘big pharma’ to be transparent on COVID-19 vaccine costs, Reuters, 29 October 2020.
- Charles Kenny, Release COVID-19 vaccine contracts, Center for Global Development, February 2021.
- According to the TI-Global Health report, the Unicef COVID-19 Vaccine Market Dashboard is ‘the most comprehensive database on COVID-19 vaccine prices and uses secondary sources such as media reports and press statements. Whilst imperfect compared to sourcing information directly from contracts, it allows for high-level trends or anomalies in prices between countries to be identified.’
- For example, in relation to the US donations, the New York Times reported (30 June 2021): ‘Officials can still run into significant hurdles. Since the donated doses were produced and sold under American legal and regulatory procedures, they have to be separately approved by the countries receiving them. The process often involves working out kinks with foreign regulators.’
- The contract between the Dominican Republic and Pfizer, for example, states that the government will ‘indemnify, defend, and hold harmless Pfizer, its partner BioNTech and its affiliates’ from costs and legal cases ‘arising out of, relating to, or resulting from the Vaccine, including but not limited to any stage of design, development, investigation, formulation, testing, clinical testing, manufacture, labelling, packaging, transport, storage, distribution, marketing, promotion, sale, purchase, licensing, donation, dispensing, prescribing, administration, provision, or use of the vaccine’. This clause goes much further than comparable clauses in other contracts or laws.
- Human Rights Watch, Covax: Enhance transparency, share intellectual property, May 2021.
- Human Rights Watch, Covax: Enhance transparency, share intellectual property, May 2021.
- Eric Sagonowsky, Moderna won’t enforce COVID-19 vaccine patents during pandemic, Fierce Pharma, October 2020.
- See A global deal for our pandemic age: Report of G20 High Level Independent Panel on Financing the Global Commons for Pandemic Preparedness and Response, June 2021.